Alternatived Products of [ 1585-40-6 ]
Product Details of [ 1585-40-6 ]
| CAS No. : | 1585-40-6 |
MDL No. : | MFCD00020281 |
| Formula : |
C11H6O10
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | QNSOHXTZPUMONC-UHFFFAOYSA-N |
| M.W : |
298.16
|
Pubchem ID : | 15314 |
| Synonyms : |
Pentacarboxybenzene
|
Application In Synthesis of [ 1585-40-6 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1585-40-6 ]
- 1
-
 [ 908094-01-9 ]
[ 908094-01-9 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 3327-06-8 ]
[ 3327-06-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With diethyl ether |
|
Reference:
[1]Alder; Rickert
[Chemische Berichte, 1937, vol. 70, p. 1354,1359]
Guiteras; Nakamiya; Inhoffen
[Justus Liebigs Annalen der Chemie, 1932, vol. 494, p. 116,122]
Feist
[Justus Liebigs Annalen der Chemie, 1932, vol. 496, p. 99,115]
- 2
-
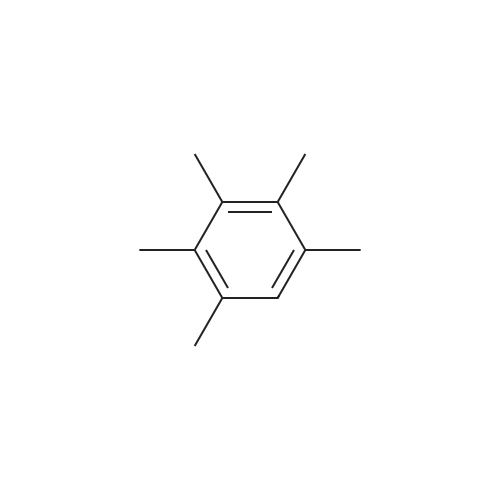 [ 700-12-9 ]
[ 700-12-9 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
| 69% |
With cerium(III) chloride; 1,1,1-trichloroethanol; oxygen In acetonitrile at 60℃; Irradiation; |
|
|
With potassium permanganate; water |
|
|
With nitric acid at 180℃; |
|
Reference:
[1]Wang, Chang-Cheng; Zhang, Guo-Xiang; Zuo, Zhi-Wei; Zeng, Rong; Zhai, Dan-Dan; Liu, Feng; Shi, Zhang-Jie
[Science China Chemistry, 2021, vol. 64, # 9, p. 1487 - 1492]
[2]Friedel; Crafts
[Annales de Chimie (Cachan, France), 1884, vol. <6> 1, p. 482][Annales de Chimie (Cachan, France), 1887, vol. <6> 11, p. 266]
[3]Freudenberg,K. et al.
[Chemische Berichte, 1962, vol. 95, p. 2814 - 2828]
- 3
-
 [ 15517-57-4 ]
[ 15517-57-4 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With potassium permanganate |
|
- 4
-
 [ 514-10-3 ]
[ 514-10-3 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
- 5
-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
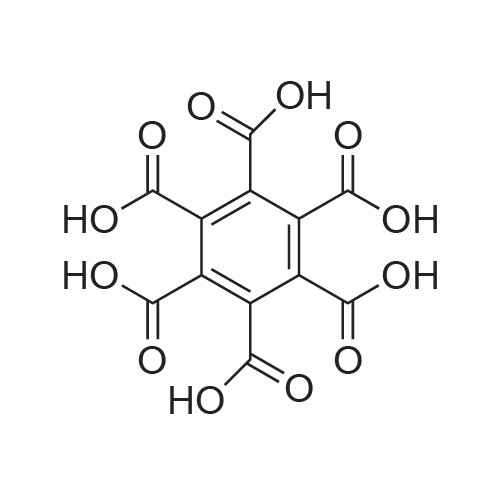 [ 517-60-2 ]
[ 517-60-2 ]

-
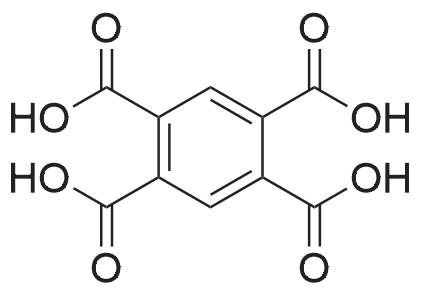 [ 89-05-4 ]
[ 89-05-4 ]
- 6
-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 59025-58-0 ]
[ 59025-58-0 ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 240 - 250℃; unter vermindertem Druck; |
|
Reference:
[1]Wolff
[Justus Liebigs Annalen der Chemie, 1902, vol. 322, p. 389]
Freund; Fleischer
[Justus Liebigs Annalen der Chemie, 1918, vol. 414, p. 30][Justus Liebigs Annalen der Chemie, 1910, vol. 373, p. 308,310]
- 7
-
 [ 872281-48-6 ]
[ 872281-48-6 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With nitric acid at 140 - 150℃; im Rohr; |
|
- 8
-
8,8-diethyl-1,2,3,3a,4,5-hexahydro-cyclopenta[<i>e</i>]acenaphthylene-7,9-dione
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
2,2-diethyl-1,3-dioxo-indan-4,5,6-tricarboxylic acid
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With nitric acid at 140℃; im Rohr; |
|
- 9
-
 [ 68106-84-3 ]
[ 68106-84-3 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With nitric acid at 160 - 170℃; im Rohr; |
|
- 10
-
 [ 872278-59-6 ]
[ 872278-59-6 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With nitric acid at 160 - 170℃; im Rohr; |
|
- 11
-
 [ 871886-21-4 ]
[ 871886-21-4 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With nitric acid at 140 - 150℃; im Rohr; |
|
- 12
-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 85304-46-7 ]
[ 85304-46-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With NaH2PO4-Na2HPO4 buffer In water for 1.38889E-05h; Irradiation; irradiation: 10 μA, 2.8 MeV electrons; |
|
- 13
-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 124-38-9 ]
[ 124-38-9 ]

-
 [ 89-32-7 ]
[ 89-32-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 270 - 300℃; |
|
| Yield | Reaction Conditions | Operation in experiment |
|
With nitric acid at 130 - 140℃; |
|
| Yield | Reaction Conditions | Operation in experiment |
|
With potassium hydroxide Oxydation des Produktes mit konz. KMnO4-Loesung; |
|
| Yield | Reaction Conditions | Operation in experiment |
|
With potassium permanganate; alkaline solution |
|
| Yield | Reaction Conditions | Operation in experiment |
|
With manganese(IV) oxide; sulfuric acid |
|
- 19
-
 [ 872281-48-6 ]
[ 872281-48-6 ]

-
 [ 7697-37-2 ]
[ 7697-37-2 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 150℃; im Rohr; |
|
- 20
-
 [ 68106-84-3 ]
[ 68106-84-3 ]

-
 [ 7697-37-2 ]
[ 7697-37-2 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 160 - 170℃; im Rohr; |
|
- 21
-
 [ 872278-59-6 ]
[ 872278-59-6 ]

-
 [ 7697-37-2 ]
[ 7697-37-2 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 160 - 170℃; |
|
- 22
-
 [ 194784-00-4 ]
[ 194784-00-4 ]

-
 [ 7697-37-2 ]
[ 7697-37-2 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 200℃; |
|
- 23
-
 [ 691364-62-2 ]
[ 691364-62-2 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 190 - 200℃; |
|
- 24
-
5-methyl-1,2,3,4,4a,7,8,9,10,11,12,12a-dodecahydro-chrysene
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
at 195 - 200℃; |
|
- 25
-
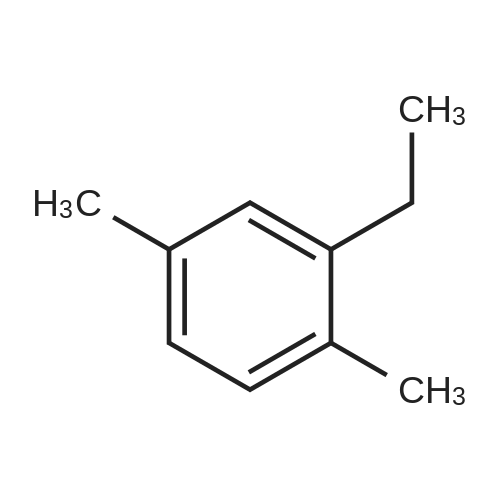 [ 1758-88-9 ]
[ 1758-88-9 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: aluminium chloride; carbon disulfide
2: nitric acid / 140 - 150 °C / im Rohr |
|
- 26
-
 [ 2142-73-6 ]
[ 2142-73-6 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: amalgamated zinc; hydrochloric acid
2: aluminium chloride; carbon disulfide
3: nitric acid / 140 - 150 °C / im Rohr |
|
- 27
-
 [ 68106-83-2 ]
[ 68106-83-2 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: concentrated sulfuric acid
2: diluted nitric acid / 160 - 170 °C / im Rohr |
|
- 28
-
3-chloro-1-(4-methyl-[1]naphthyl)-butan-1-one
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: concentrated sulfuric acid
2: diluted nitric acid / 160 - 170 °C / im Rohr |
|
- 29
-
 [ 90-12-0 ]
[ 90-12-0 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: aluminium chloride; carbon disulfide
2: diluted nitric acid / 160 - 170 °C / im Rohr |
|
|
Multi-step reaction with 3 steps
1: aluminium chloride; carbon disulfide
2: concentrated sulfuric acid
3: diluted nitric acid / 160 - 170 °C / im Rohr |
|
|
Multi-step reaction with 3 steps
1: aluminium chloride; carbon disulfide
2: concentrated sulfuric acid
3: diluted nitric acid / 160 - 170 °C / im Rohr |
|
- 30
-
{Co(NH3)5(H2O)}(3+)*3OH(1-)={Co(NH3)5(H2O)}(OH)3
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
2Na(1+)*Co(3+)*5NH3*C6H(CO2)5(5-)=Na2{Co(NH3)5(C6H(CO2)5)}
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
reaction of {Co(NH3)5(H2O)}(OH)3 with an alkali-salt of benzene pentacarbonate;; |
|
|
reaction of {Co(NH3)5(H2O)}(OH)3 with an alkali-salt of benzene pentacarbonate;; |
|
- 31
-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 7732-18-5 ]
[ 7732-18-5 ]

-
 [ 1310-73-2 ]
[ 1310-73-2 ]

-
 [ 7646-85-7 ]
[ 7646-85-7 ]

-
[Zn3(benzenepentacarboxylato)(OH)(H2O)3]
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 30% |
In water High Pressure; mixt. of Zn salt, benzenepentacarboxylic acid, and NaOH in H2O (final pHca. 4.0) heated at 125°C in autoclave for 2 d; crystals filtered off, dried on air; |
|
- 32
-
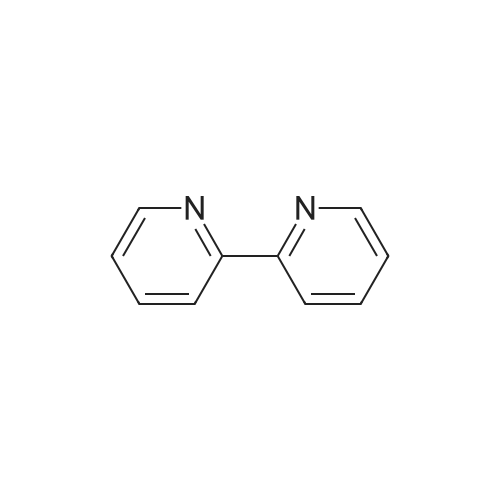 [ 366-18-7 ]
[ 366-18-7 ]

-
zinc(II) nitrate hexahydrate
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 1310-73-2 ]
[ 1310-73-2 ]

-
[Zn5(μ3-OH)2(1,2,3,4,5-benzenepentacarboxylic acid(-4H))2(2,2'-bipyridyl)2]
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 75% |
In water High Pressure; under hydrothermal conditions; mixt. of Zn salt, ligand, 2,2'-bipyridine, NaOH and H2O (molar ratio 4:1:2:4:300) stirred; heated in stainless steel reactor at 175°C for 60 h; cooled (5°C/h); filtered; crystals washed with H2O; air dried; elem. anal.; |
|
Reference:
[1]Wang, Jing; Lin, Zhuojia; Ou, Yong-Cong; Yang, Nai-Liang; Zhang, Yue-Hua; Tong, Ming-Liang
[Inorganic Chemistry, 2008, vol. 47, # 1, p. 190 - 199]
- 33
-
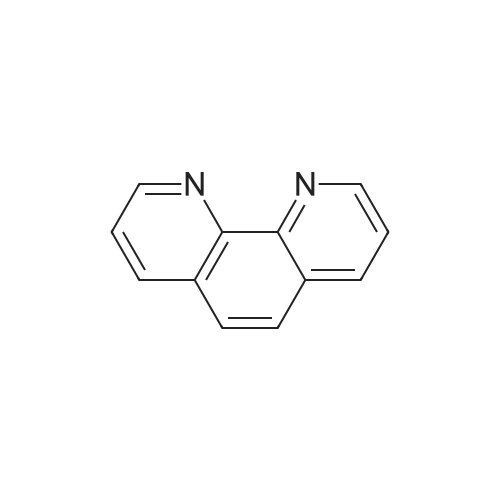 [ 66-71-7 ]
[ 66-71-7 ]

-
zinc(II) nitrate hexahydrate
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 1310-73-2 ]
[ 1310-73-2 ]

-
[Zn5(1,2,3,4,5-benzenepentacarboxylate)2(1,10-phenanthroline)4(H2O)3]*2H2O
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 40% |
In water High Pressure; under hydrothermal conditions; mixt. of Zn salt, ligand, phenanthroline,NaOH (molar ratio 4:1:2:8) stirred; heated in stainless steel reactor a t 140°C for 120 h; cooled (5°C/h); crystals mechanically sepd.; washed with H2O and abs. EtOH; air dried; elem. anal.; |
|
Reference:
[1]Wang, Jing; Lin, Zhuojia; Ou, Yong-Cong; Yang, Nai-Liang; Zhang, Yue-Hua; Tong, Ming-Liang
[Inorganic Chemistry, 2008, vol. 47, # 1, p. 190 - 199]
- 34
-
 [ 66-71-7 ]
[ 66-71-7 ]

-
zinc(II) nitrate hexahydrate
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 1310-73-2 ]
[ 1310-73-2 ]

-
[Zn2(1,2,3,4,5-benzenepentacarboxylic acid(-4H))(1,10-phenanthroline)2(H2O)2]
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 60% |
In water High Pressure; under hydrothermal conditions; mixt. of Zn salt, ligand, phenanthroline,NaOH (molar ratio 4:1:2:4) stirred; heated in stainless steel reactor a t 140°C for 60 h; cooled (5°C/h); crystals mechanically sepd.; washed with H2O and abs. EtOH; air dried; elem. anal.; |
|
Reference:
[1]Wang, Jing; Lin, Zhuojia; Ou, Yong-Cong; Yang, Nai-Liang; Zhang, Yue-Hua; Tong, Ming-Liang
[Inorganic Chemistry, 2008, vol. 47, # 1, p. 190 - 199]
- 35
-
zinc(II) nitrate hexahydrate
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 1310-73-2 ]
[ 1310-73-2 ]

-
[Zn6(μ3-OH)2(1,2,3,4,5-benzenepentacarboxylate)2(H2O)6]
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 40% |
In water High Pressure; under hydrothermal conditions; mixt. of NaOH in H2O and ligand in H2O added to aq. soln. of Zn salt (molar ratio 8:1:4); stirred; heated in stainless steel reactor at 175°C for 90 h; cooled (5°C/h); filtered; crystals washed with H2O; air dried; elem. anal.; |
|
Reference:
[1]Wang, Jing; Lin, Zhuojia; Ou, Yong-Cong; Yang, Nai-Liang; Zhang, Yue-Hua; Tong, Ming-Liang
[Inorganic Chemistry, 2008, vol. 47, # 1, p. 190 - 199]
- 36
-
cobalt(II) chloride hexahydrate
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 1310-73-2 ]
[ 1310-73-2 ]

-
[Co3(benzenepentacarboxylato)(OH)(H2O)3]
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 70% |
In water High Pressure; mixt. of Co salt (0.25 mmol), benzenepentacarboxylic acid (0.10 mmol), and NaOH (0.5 mmol) in H2O (final pH ca. 5.0) heated at 195°C in autoclave for 3 d; crystals filtered off, washed with H2O, dried on air; |
|
- 37
-
cobalt(II) chloride hexahydrate
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
[Co5(benzenepentacarboxylato)2(H2O)12]*(H2O)12
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 15% |
With NaOH In water High Pressure; mixt. of Co salt (0.75 mmol), benzenepentacarboxylic acid (0.15 mmol), and NaOH (0.7 mmol) in H2O (final pH ca. 4.0) heated at 135°C in autoclave for 3 d; crystals manually sepd. from powder, washed with H2O; |
|
- 38
-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
benzenepentacarboxylic pentaacid chloride
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With phosphorus pentachloride at 150℃; for 24h; |
|
Reference:
[1]Location in patent: experimental part
Yang, Chih-Chiang; Hsu, Chia-Jung; Chou, Pi-Tai; Cheng, Hsu Chun; Su, Yuhlong Oliver; Leung, Man-Kit
[Journal of Physical Chemistry B, 2010, vol. 114, # 2, p. 756 - 768]
- 39
-
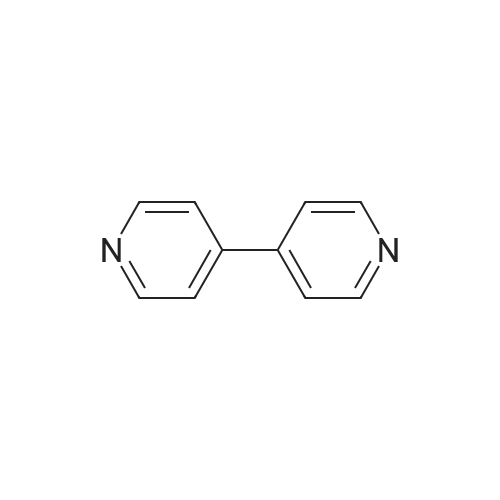 [ 553-26-4 ]
[ 553-26-4 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
2C10H8N2*C11H6O10
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 66% |
In water at 139.84℃; for 96h; High pressure; Autoclave; |
Molecular complex [(BPC)(bpy)2], 1b:
BPC (74.5mg, 0.25mmol) and bpy (39.0mg, 0.25mmol) and water (15mL) were placed in a Teflon-lined stainless steel vessel under autogeneous pressure and heated up to 413K for 4days and subsequently cooled to room temperature. Colorless crystalline product was collected and used for X-ray diffraction analysis. Crystal were isolated by filtration and allowed to dry in air to give 40mg (yield is 66%, based on the formula weight of 1b). (0016) Under hydrothermal condition, supramolecular reactions between BPC and bpy were carried out in 1:1, 1:2 and 2:1 ratio at 413K for 4days. The reaction mixtures were cooled and subsequent slow evaporation at room temperature yields a 2:1 complex irrespective of the ratio of the components used (which is been confirmed by the unit cell collection of many crystals from the reaction mixture using Single crystal XRD). |
- 40
-
 [ 553-26-4 ]
[ 553-26-4 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
C10H8N2*2C11H6O10
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 57% |
In water at 26.84℃; for 96h; |
Molecular complex, [(BPC)2(bpy)(H2O)7], 1a:
BPC (74.5mg, 0.25mmol) and 4,4′-bipyridine, bpy (39.0mg, 0.25mmol) were dissolved in water (15mL) with stirring. Upon slow evaporation of the solvent, colorless blocks of single crystals suitable for X-ray diffraction analysis were obtained in 4days. Crystal were isolated by filtration and allowed to dry in air to give 50mg (yield is 57%, based on the formula weight of 1a.). Different ratio of components of BPC and bpy such as 2:1, 3:1, 4:1, 5:1 and 1:2 were co-crystallized from water at room temperature through slow evaporation process. Colorless crystals were obtained after complete evaporation of the solvent. Different morphological crystals were isolated by hand picking and many of them are subjected to unit cell collection in single crystal XRD. |
- 41
-
 [ 553-26-4 ]
[ 553-26-4 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
C10H8N2*C11H6O10
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 20% |
With nickel(II) nitrate hexahydrate In water at 139.84℃; for 72h; High pressure; Autoclave; |
Molecular complex [(BPC)(bpy)(H2O)2], 1c:
BPC (74.5mg, 0.25mmol) and bpy (39.0mg, 0.25mmol), Ni(NO3)2.6H2O (29.1mg, 0.1mmol) and water (15mL) was placed in a Teflon-lined stainless steel vessel under autogeneous pressure, which is heated to 413K for 72h and subsequently cooled to room temperature. Colorless crystalline product was collected used for X-ray diffraction analysis. Crystal were isolated by filtration and allowed to dry in air to give 10mg (yield is 20%, based on the formula weight of 1c). (0018) Supramolecular synthesis has been carried out under hydrothermal condition using BPC (74.5mg, 0.25mmol) and bpy (39.0mg, 0.25mmol) along with Mn(II)/Zn(II) (0.1mmol /0.1mmol) at 413K for 3days. The reaction mixture is cooled to room temperature and allowed to evaporate slowly. The solid obtained after complete evaporation of the solvent is characterized through single crystal X-ray diffraction technique. |
- 42
-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 36752-26-8 ]
[ 36752-26-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With dihydrogen peroxide; sodium hydroxide for 1h; Alkaline conditions; |
|
- 43
-
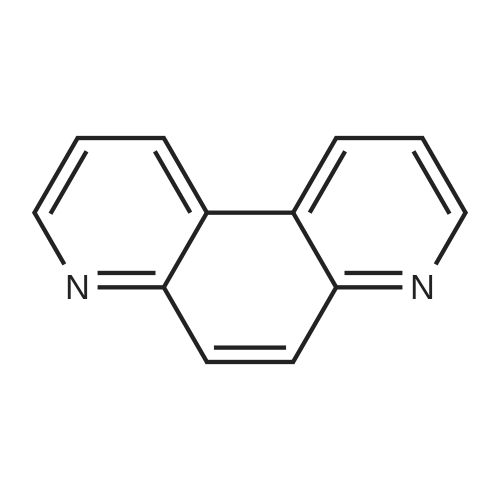 [ 230-07-9 ]
[ 230-07-9 ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
C11H6O10*C12H8N2
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In dimethyl sulfoxide; |
General procedure: All chemicals used in this study were obtained from commercial suppliers (Sigma-Aldrich) and were used without any further purification. The spectroscopic grade solvents employed for the crystallization purpose were of the highest available purity. All the molecular complexes were prepared by dissolving <strong>[230-07-9]4,7-phenanthroline</strong> (1) and the corresponding carboxylic acids in a solution of dimethylsulfoxide (DMSO), as listed in Chart 1, in a 1:1 ratio and then by slow evaporation of the solution at ambient conditions. Single crystals were obtained within 48h in all the cases. In a typical example, 18.3mg (0.97mmol) of 1 and 16.6mg (0.99mmol) of pyridine-2,6-dicarboxylic acid, a, were dissolved by heating up to 80C in 2mL DMSO and allowed for slow evaporation at ambient conditions. Good quality single crystals suitable for X-ray diffraction study were obtained in 48h. |
- 44
-
 [ 4916-57-8 ]
[ 4916-57-8 ]

-
cadmium(II) acetate monohydrate
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
0.5C2O4(2-)*3H2O*C11HO10(5-)*2Cd(2+)*1.5C12H12N2*2H(1+)
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In water at 140℃; for 96h; Autoclave; High pressure; |
2.2. Preparation of the complexes
General procedure: The complexes were prepared by hydrothermal method by carrying out reactions in a 50 mL Teflon-lined autoclave at a neutral pH. In a typical example, complex 1a was prepared by placing a mixture of Co(NO3)2·6H2O (29.1 mg, 0.1 mmol), BPCH5 (29.8 mg, 0.1 mmol) and bpy (15.6 mg, 0.1 mmol) that was kept in a Teflon flask with about 15 mL of water in an autoclave and heated at 140 °C for 4 days. The resultant neutral solution was allowed for slow evaporation over a period of 10 day at ambient conditions. The obtained crystalline compound was collected by filtration, washed with water and dried in air. The washed compounds contained diffraction quality block shaped single crystals. |
- 45
-
 [ 553-26-4 ]
[ 553-26-4 ]

-
copper(II) nitrate trihydrate
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
C11HO10(5-)*2Cu(2+)*C10H8N2*4H2O*H(1+)
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In water at 140℃; for 96h; Autoclave; High pressure; |
2.2. Preparation of the complexes
General procedure: The complexes were prepared by hydrothermal method by carrying out reactions in a 50 mL Teflon-lined autoclave at a neutral pH. In a typical example, complex 1a was prepared by placing a mixture of Co(NO3)2·6H2O (29.1 mg, 0.1 mmol), BPCH5 (29.8 mg, 0.1 mmol) and bpy (15.6 mg, 0.1 mmol) that was kept in a Teflon flask with about 15 mL of water in an autoclave and heated at 140 °C for 4 days. The resultant neutral solution was allowed for slow evaporation over a period of 10 day at ambient conditions. The obtained crystalline compound was collected by filtration, washed with water and dried in air. The washed compounds contained diffraction quality block shaped single crystals. |
- 46
-
 [ 4916-57-8 ]
[ 4916-57-8 ]

-
copper(II) nitrate trihydrate
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
Cu(2+)*C11HO10(5-)*C12H12N2*2H2O*3H(1+)
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In water at 140℃; for 96h; Autoclave; High pressure; |
2.2. Preparation of the complexes
General procedure: The complexes were prepared by hydrothermal method by carrying out reactions in a 50 mL Teflon-lined autoclave at a neutral pH. In a typical example, complex 1a was prepared by placing a mixture of Co(NO3)2·6H2O (29.1 mg, 0.1 mmol), BPCH5 (29.8 mg, 0.1 mmol) and bpy (15.6 mg, 0.1 mmol) that was kept in a Teflon flask with about 15 mL of water in an autoclave and heated at 140 °C for 4 days. The resultant neutral solution was allowed for slow evaporation over a period of 10 day at ambient conditions. The obtained crystalline compound was collected by filtration, washed with water and dried in air. The washed compounds contained diffraction quality block shaped single crystals. |
- 47
-
 [ 553-26-4 ]
[ 553-26-4 ]

-
cobalt(II) nitrate hexahydrate
[ No CAS ]

-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
C11HO10(5-)*1.5C10H8N2*Co(2+)*6H2O*3H(1+)
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In water at 140℃; for 96h; Autoclave; High pressure; |
2.2. Preparation of the complexes
The complexes were prepared by hydrothermal method by carrying out reactions in a 50 mL Teflon-lined autoclave at a neutral pH. In a typical example, complex 1a was prepared by placing a mixture of Co(NO3)2·6H2O (29.1 mg, 0.1 mmol), BPCH5 (29.8 mg, 0.1 mmol) and bpy (15.6 mg, 0.1 mmol) that was kept in a Teflon flask with about 15 mL of water in an autoclave and heated at 140 °C for 4 days. The resultant neutral solution was allowed for slow evaporation over a period of 10 day at ambient conditions. The obtained crystalline compound was collected by filtration, washed with water and dried in air. The washed compounds contained diffraction quality block shaped single crystals. |
- 48
-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 108-46-3 ]
[ 108-46-3 ]

-
C35H18O12
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 1.88 g |
Stage #1: benzenepentacarboxylic acid In tetrahydrofuran at 140℃; for 24h; Irradiation;
Stage #2: recorcinol With methanesulfonic acid In tetrahydrofuran at 90℃; for 48h; |
4.A
Benzene pentacarboxylic acid (894 mg) was suspended in tetrahydrofuran, the powder was pulverized by ultrasonic waves, and the solvent was distilled off under reduced pressure. The resulting powder was heated at 140 ° C. for 24 hours. After returning to room temperature under reduced pressure, resorcinol (1.32 g) and methanesulfonic acid (10 mL) were added and the mixture was stirred at 90 ° C. for 2 days. After returning the reaction solution to room temperature, it was gradually added to ice-cooled water (100 mL) with stirring. The precipitated solid was collected by filtration, washed with water and lyophilized to obtain a deep red powder (1.88 g). This was refluxed with acetic anhydride (14 mL) for 1 day. After distilling off the solvent of the reaction solution under reduced pressure, it was purified three times by silica gel column chromatography (first and second times; dichloromethane / methanol = 95/5 → 93/7 → 90/10, third time; ethyl acetate / Methanol = 100/0 -> 95/5 -> 90/10) to obtain the target compound 2 'as a pale yellow powder (1.6 g, yield 67%). |
- 49
-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
1,2,3,5,6-benzenepentacarboxamide
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With ammonia derivative at 290℃; for 0.5h; |
amide intermediate preparation examples
General procedure: In 1L reactor was added 500g of carboxylic acid raw material (chemically pure), And charged with 1.3 moles of ammonia (water content: 0.5% by weight, industrial product) having a mole number of the carboxyl group contained in the carboxylic acid raw material, Or ammonium hydrogen carbonate powder (chemically pure) having a mole number of ammonium ions of 1.4 times the carboxyl group contained in the carboxylic acid starting material, The reaction kettle was closed and the stirrer was turned on (600r / min). After the reaction was allowed to proceed at a reaction temperature of TA for TC hours, The contents of the reactor were sampled and subjected to 1H NMR and elemental analysis to characterize the amide intermediate. The specific reaction conditions and characterization results are shown in Table A-1, Table A-2, Table A-3 and Table A-4 below. These characterization results indicate that the amide intermediate obtained has an extremely high purity (99% or more). |
|
With ammonium for 0.5h; Heating; |
Amide Intermediate Preparation Example
General procedure: In a 1L reactor, 500 g of a carboxylic acid feed (chemically pure) and NH3 moles were added to the carboxyl groups of the carboxylic acid starting material.1.4 times of ammonia (NH3 content is 25wt%, industrial product) or the number of moles of ammonium ion is 1.6 of the carboxyl group contained in the carboxylic acid raw material.The aqueous solution of ammonium hydrogencarbonate (having a concentration of ammonium bicarbonate of 30 wt%) was sealed and the reaction vessel was closed and the stirring was turned on (600 r/min). Make the reactionAfter TC hours at the reaction temperature Τα, the contents of the reactor were sampled for nuclear magnetic resonance and elemental analysis to characterize the acyl group.Amine intermediates. Specific reaction conditions and characterization results are shown in Table A-1, Table A-2, Table A-3 and Table A-4 below. These characterization resultsIt shows that the obtained amide intermediate has a very high purity (above 99%). |
|
With ammonia at 295℃; for 0.5h; |
Amide Intermediate Preparation Example
General procedure: 500 g of carboxylic acid raw material (chemically pure) was added to a 1 L open reactor and stirring was turned on (600 r/min).Ammonia gas was continuously introduced into the carboxylic acid feed from the bottom of the reactor (chemical purity, water content 5.1 wt%, flow rate 100 g/min). After the reaction was allowed to proceed for TC hours at the reaction temperature TA, ammonia gas flow was stopped. The contents of the reactor were sampled and nuclear magnetic proton spectra and elemental analysis were performed to characterize the amide intermediate. Specific reaction conditions and characterization results are shown in Table A-1, Table A-2, Table A-3 and Table A-4 below. These characterization results show that the amide intermediates obtained have a very high purity (above 99%). |
- 50
-
 [ 1585-40-6 ]
[ 1585-40-6 ]

-
 [ 38700-21-9 ]
[ 38700-21-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: ammonia derivative / 0.5 h / 290 °C
2: 1 h / 345 °C |
|
|
Multi-step reaction with 2 steps
1: ammonium / 0.5 h / Heating
2: phosphorus pentoxide / 1 h / 15 - 37.5 Torr / Heating |
|
|
Multi-step reaction with 2 steps
1: ammonia / 0.5 h / 295 °C
2: phosphorus pentoxide / 1 h / 345 °C / 15 - 37.5 Torr |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping















