| 100% |
With sodium hydrogencarbonate; In dichloromethane; at 0℃; for 2h; |
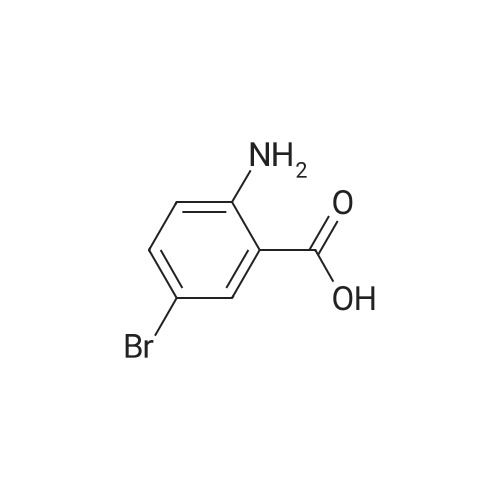
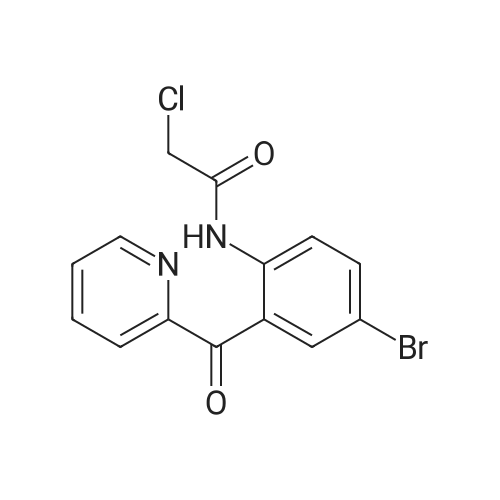
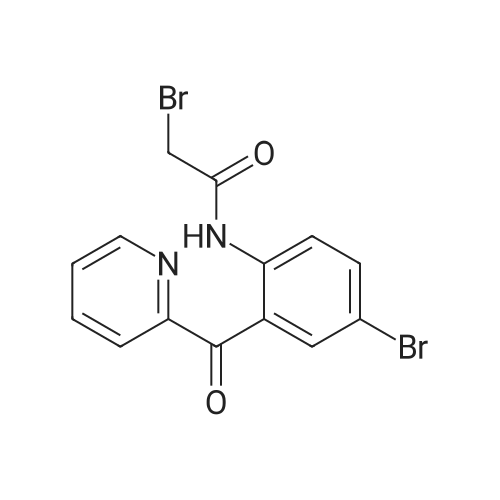
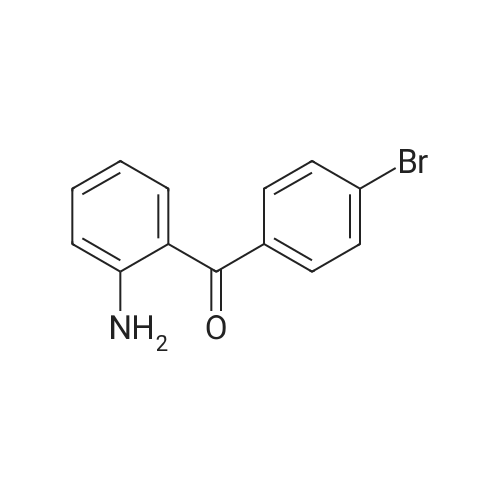
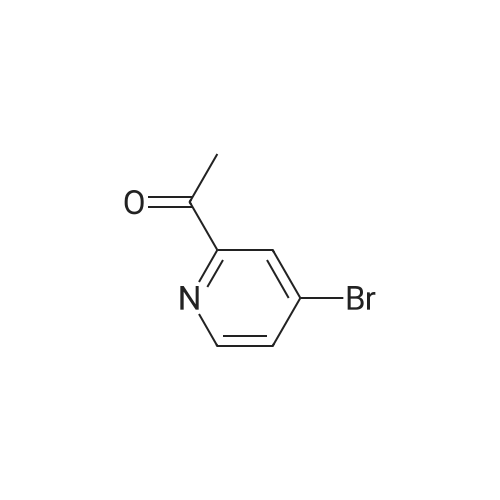
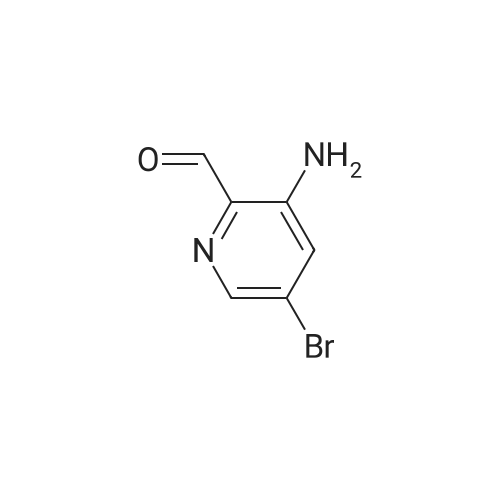
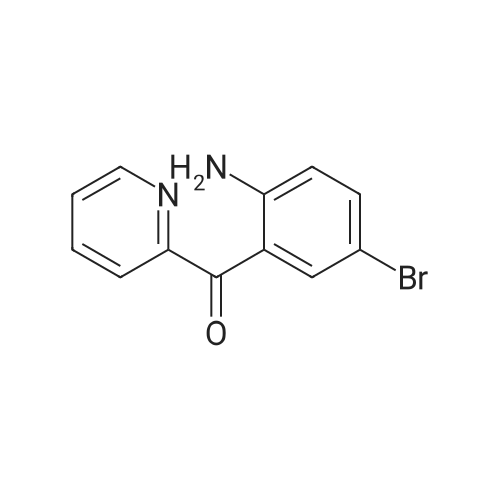
2-(2-Amino-5-bromobenzoyl)pyridine (compound 1a, 60 g, 0.22 mol) was dissolved in DCM (3 L), and NaHCO3 (36.9 g, 0.44 mol) was added. The mixture was cooled to 0 C., and 2-bromoacetyl bromide (52.4 g, 0.26 mol) was added dropwise slowly. The reaction mixture was stirred at 0 C. for 2 h until TLC indicated that the reaction was completed. The reaction mixture was concentrated to obtain 2-bromo-N-(4-bromo-2-(pyridin-2-ylcarbonyl)phenyl)acetamide (compound 1b, 88 g, yield: 100.0%). |
|
With sodium hydrogencarbonate; In dichloromethane; at 0 - 20℃; |
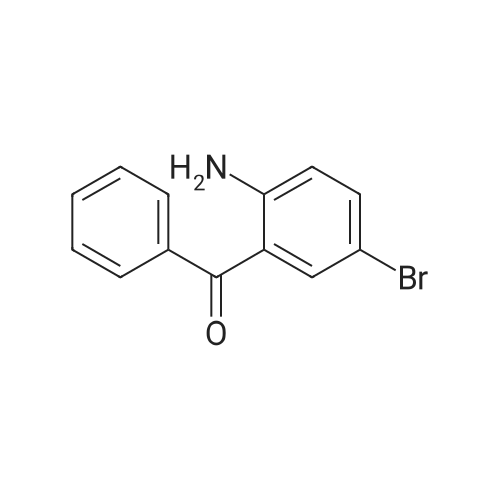
The 2?-pyridyl ketone 5 (120 g, 433.2 mmol) was dissolved in anhydDCM (1.4 L) in a 3-necked round-bottom flask and stirred with anoverhead mechanical stirrer for 15 min to obtain a homogenous solution.Then solid NaHCO3 (72.8 g, 866.4 mmol) was added to the solutionwith vigorous stirring to avoid clogging of the stirrer, and themixture was cooled to 0 C using an ice bath. A solution of bromoacetylbromide (144.9 g, 714.8 mmol) in anhyd DCM (200 mL) was thenadded dropwise at 0 C with an addition funnel. The reaction mixturewas allowed to warm to r.t. and stirred overnight until the startingmaterial was consumed as monitored on analysis by TLC (silica gel,EtOAc/hexanes 1:1). The mixture was quenched with ice-water (500mL) and stirred at r.t. for 30 min. The organic layer was then separated,and the aqueous layer was extracted with DCM (3 × 250 mL). Theorganic layers were combined, washed (200 mL each) sequentiallywith sat. aq NaHCO3, H2O, 10% HCl, brine, and then dried (Na2SO4).The solvent was then concentrated to one quarter of its original volumeunder reduced pressure. The intermediate 6, which was prepared,was used in the next step without further purification.MeOH (1.4 L) was cooled to 0 C using an ice-water cooling bath andsaturated with anhyd ammonia gas. The DCM solution of the aboveprepared intermediate 6 was added to the solution of saturatedMeOH/NH3 at 0 C. The mixture was allowed to warm to r.t. and slowlyheated to reflux (Caution mild exotherm observed) until the startingmaterial was consumed on analysis by TLC (silica gel) in 12 h. Themixture was then cooled to r.t. and the solvent was removed underreduced pressure. The solid which remained was filtered and washedwith H2O (3 × 150 mL), cold EtOAc (3 × 50 mL), and DCM (3 × 50 mL).The crude solid was dissolved in MeOH (600 mL) and DCM (100 mL)at 60 C, and the solution was concentrated to one quarter of its originalvolume. The amide 7 was recrystallized from MeOH and DCM atr.t. and filtered, after which it was washed with DCM to obtain themajority of the pure amide 7 as white crystals. The filtrates werecombined and concentrated under reduced pressure to an oily residue,which was further purified by flash chromatography on silica gel(EtOAc/hexanes 1:1 and 1% of Et3N) to afford additional amide 7;yield: 108.6 g (78% over the two steps); mp 228-229 C; Rf = 0.4 (EtOAc/hexanes 1:1 and 1% of Et3N).1H NMR (300 MHz, DMSO-d6): delta = 10.63 (s, 1 H), 8.55 (d, J = 4.1 Hz, 1H), 8.04 (d, J = 7.7 Hz, 1 H), 7.93 (d, J = 7.4 Hz, 1 H), 7.69 (d, J = 8.6 Hz,1 H), 7.54-7.44 (m, 1 H), 7.42 (s, 1 H), 7.18 (d, J = 8.7 Hz, 1 H), 4.23 (s,2 H).13C NMR (75 MHz, DMSO-d6): delta = 170.3, 168.1, 156.3, 148.9, 139.3,137.5, 134.4, 134.1, 127.9, 125.4, 123.9, 123.6, 114.5, 57.6.HRMS (ESI/IT-TOF): m/z [M + H]+ calcd for C14H11BrN3O: 316.0080;found: 316.0076. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping