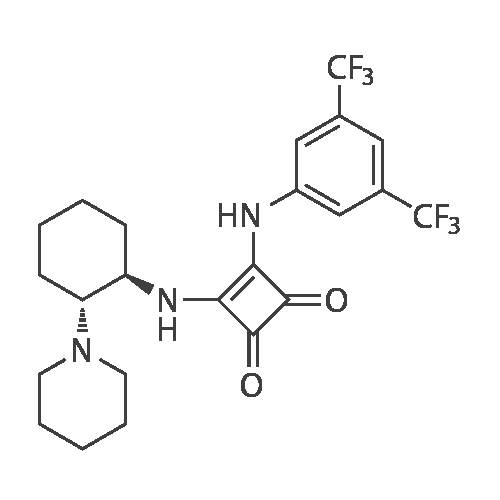
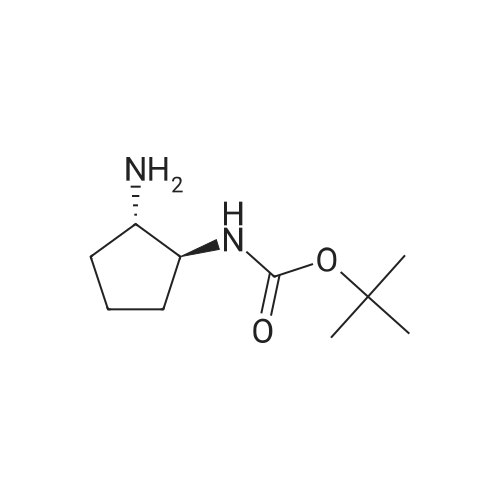
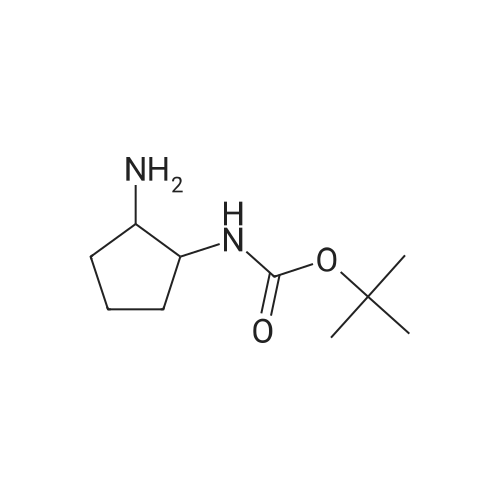
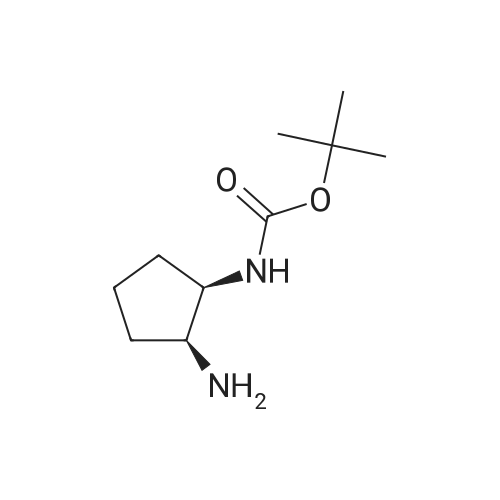
| 88% |
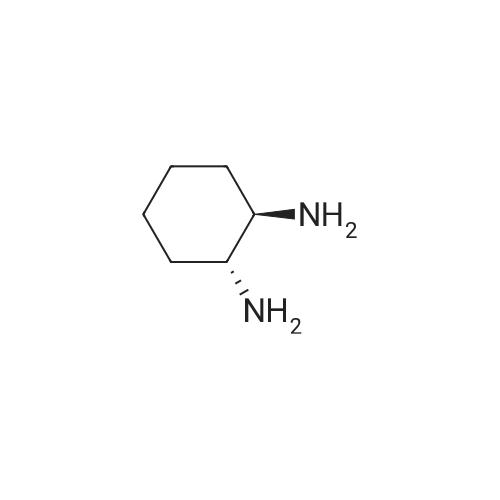
Stage #1: (1R,2R)-1,2-diaminocyclohexane With hydrogenchloride In methanol at 0℃; for 0.5h;
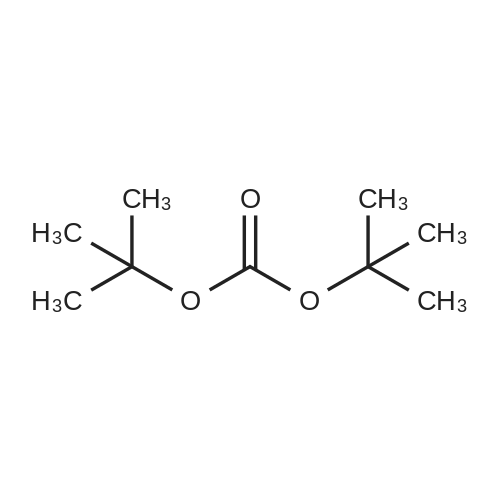
Stage #2: di-<i>tert</i>-butyl dicarbonate In methanol at 0 - 25℃; for 4h; |
4.2. Synthesis of mono-protected carbamates (5-10)
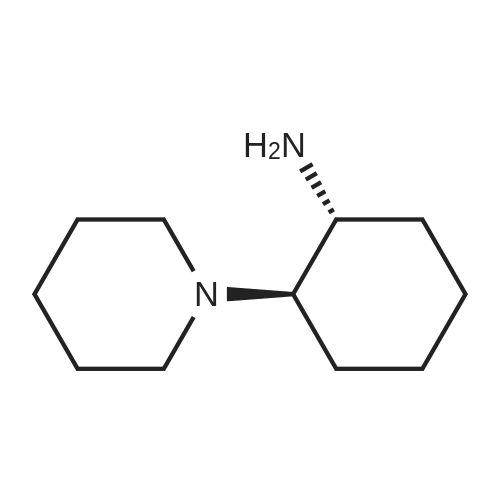
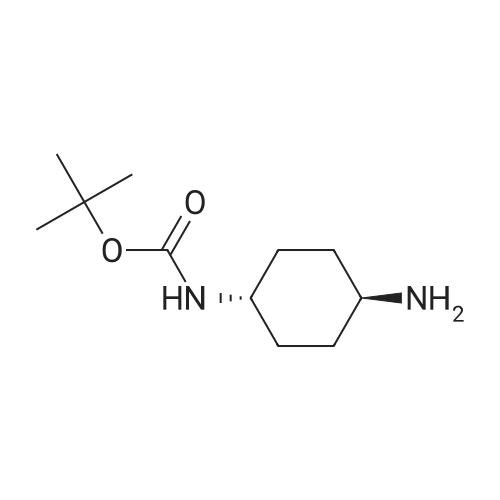
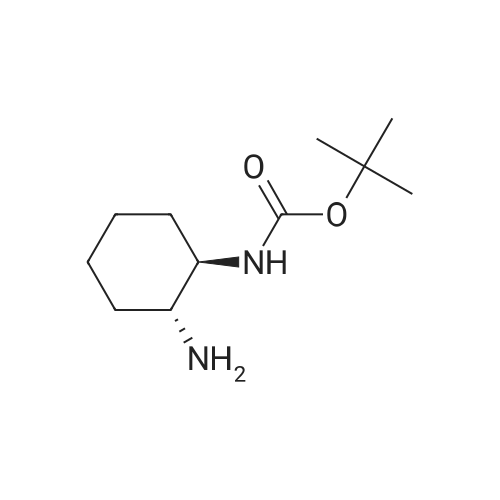
4.2.1. tert-Butyl ((1R,2R)-2-aminocyclohexyl)carbamate (5) Prepared according to the modified literature procedure [19]. (1R,2R)-DACH (780 mg, 6.83 mmol, 1.0 equiv.) was dissolved in anhydrous MeOH (15 mL) and cooled via ice bath. 37% HCl (600 mL, 7.18 mmol, 1.05 equiv.) was diluted with 5 mL anhydrous MeOH, and it was added to the reaction mixture dropwise at 0 °C. The mixture was allowed to warm up, and it was stirred for 30 min. Then, di-tert-butyl dicarbonate (2.22 g, 10.25 mmol, 1.50 equiv.) in anhydrous MeOH (10 mL) was added dropwise at 0 °C. The mixture was stirred at room temperature for 4 h. After evaporation of the MeOH, water was added, and the insoluble byproduct was filtered off. The aqueous layer was basified with 2 M NaOH, extracted with CH2Cl2 (4x), dried over Na2SO4 and concentrated in vacuo, affording compound 5 as a white solid, which was found to be pure without further purification (1.29 g, 88% yield). 1H NMR (400 MHz, CDCl3)d: 4.43 (brs, 1H, NHCOO), 3.13 (brs, 1H, CH-NHCOO), 2.32 (td, J = 10.2 Hz, 1H, CH-NH2), 1.98 (t, J = 6.0 Hz, 2H, CH2-CH), 1.70 (d, J = 6.02 Hz, 2H, CH2-CH), 1.45-1.40 (m, 11H, C-(CH3)3, CH-NH2), 1.28-1.07 (m, 4H, CH2-CH2); 13C NMR (100 MHz, CDCl3)d: 156.3 (NHCOO), 79.4 (C-(CH3)3), 57.9 (CH-NHCOO), 55.8 (CH-NH2), 35.4 (CH2-CH), 33.0 (CH2-CH), 28.5 (C-(CH3)3), 25.3 (CH2-CH2), 25.2 (CH2-CH2). |
| 85% |
In 1,4-dioxane at 20℃; for 5h; |
|
| 85% |
Stage #1: (1R,2R)-1,2-diaminocyclohexane With methanol; chloro-trimethyl-silane at 0 - 20℃; for 0.25h;
Stage #2: di-<i>tert</i>-butyl dicarbonate at 20℃; for 1.66667h; |
|
| 82% |
In dichloromethane for 24h; |
|
| 82% |
In dichloromethane for 24h; |
|
| 82% |
In dichloromethane for 24h; |
|
| 81% |
In dichloromethane at 20℃; |
|
| 81.5% |
In dichloromethane at 0 - 20℃; for 24h; Inert atmosphere; |
Synthesis of intermediate 1
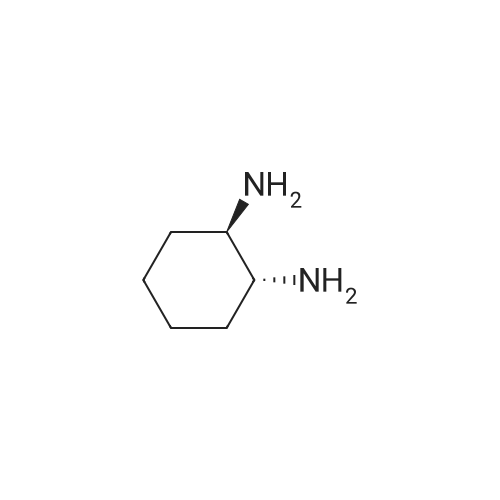
Under an atmosphere of Ar, a solution of di-tert-butyl dicarbonate (Boc2O, 4.37g, 0.02 mol) in CH2Cl2 (50 mL) was added dropwise to the solution of(1R,2R)-cyclohexane-1,2-diamine (6.85 g, 0.06 mol) in CH2Cl2 (60 mL) over a periodof 30 min at 0 oC. Then the reaction mixture was warmed to room temperature andwas stirred an additional 24 h. Water (30 mL) and CH2Cl2 (25 mL) were added todissolve the white precipitate. The organic phase was separated and concentratedunder reduced pressure. The residue was dissolved in ether (50 mL) and water (80mL). HCl (4 M) was sued to acidify the mixture to pH 5, and the aqueous wascollected. The aqueous was alkalified to pH 11 with NaOH (4 M) and extracted withEtOAc (5 X 50 mL). The organic phase was then dried and concentrated to yield theintermediate 1 (3.49 g, 81.5% yield). |
| 80% |
With hydrogenchloride In methanol at 20℃; for 1h; |
|
| 78% |
Stage #1: (1R,2R)-1,2-diaminocyclohexane With hydrogenchloride In methanol; water at 20℃; for 0.5h; Cooling with ice;
Stage #2: di-<i>tert</i>-butyl dicarbonate In methanol; water at 20℃; for 1h; |
1.1 (1)Preparation of 1-N-Boc-1R, 2R-cyclohexanediamine
22.4 g (0.1 mol) of 1R, 2R-cyclohexanediamine was dissolved in 200 mL of a methanol solution,Placed in 500mL single-necked flask at room temperature and stirring constantly;The amount of cylinder to take 5.17mol / L of methanol 19.4mL placed in a constant pressure funnel;Hydrochloric acid under ice bath slowly dropping to a single-necked flask;Dropwise.Remove the ice bath,The reaction at room temperature for 30 minutes.Weigh 38.28g (0.176mol) (Boc) 2O dissolved in 100mL of methanol,Placed in a constant pressure funnel,Slowly added to a single-necked flask,Dropwise.1 hour at room temperature,Spin dry,Obtained as a white solid;The white solid was dissolved in water,Ether extraction,Take the water phase;Adjust the pH to 10,CH2Cl2 extraction,Take the organic phase,Spin dry,CH2Cl2 / PE recrystallization,Got white crystals,Yield 78%. |
| 78% |
Stage #1: (1R,2R)-1,2-diaminocyclohexane With hydrogenchloride In methanol at 20℃; for 0.5h; Cooling with ice;
Stage #2: di-<i>tert</i>-butyl dicarbonate In methanol at 20℃; for 1h; |
1.1 1. preparation 1 - N - Boc - 1R, 2R - cyclohexyl diamine
he 22.4g (0.1 µM) 1R, 2R - cyclohexyl diamine dissolved in 200 ml of methanol solution, under the normal temperature condition is placed in the 500 ml of shan Jingping and continuously stirring; graduated cylinder quantity taking 5.17 mol/L hydrochloric acid methanol 19.4 ml is placed in the constant pressure funnel; ice bath slowly dropping hydrochloric acid methanol under the condition applied to the bottle-neck; then completing, removed ice, the reaction at room temperature 30 minutes. Weighing 38.28g (0.176 µM) (Boc)2O dissolved in 100 ml methanol, is placed in the constant pressure funnel, slowly to the shan Jingping, then completing, the reaction at room temperature 1 hour, turns on lathe does, to obtain white solid; the white solid soluble in water, ether, takes the aqueous phase; adjusting the pH to 10, CH2Cl2Extraction, takes organically phase, turns on lathe does, CH2Cl2Recrystallization/PE, to obtain white crystal, yield 78%. |
| 78% |
Stage #1: (1R,2R)-1,2-diaminocyclohexane With hydrogenchloride In methanol; water at 0 - 20℃;
Stage #2: di-<i>tert</i>-butyl dicarbonate In methanol; water at 20℃; for 2h; |
|
| 74% |
In ethanol at 20℃; for 16h; |
|
| 72% |
Stage #1: (1R,2R)-1,2-diaminocyclohexane With hydrogenchloride In methanol at 0 - 20℃; for 0.25h;
Stage #2: di-<i>tert</i>-butyl dicarbonate In methanol; water at 20℃; for 1h; |
Synthesis of (R,R)-(2-Amino-cyclohexyl)-carbamic acid tert-butyl ester
This compound is known but not fully characterised. A solution of 3.0 cm3 of HCl in MeOH (3M) was stirred at 0 °C for 15 min. Then, to this solution, was carefullyadded (R,R)-1,2-diaminocyclohexane (0.5 g, 4.36 mmol) at 0 °C. The mixture was stirred for 15 min at r.t. before adding water (1.0 cm3) and stirring for another 0.5 h.To the solution, (Boc)2O (0.95 g, 8.76 mmol) in 4.0 cm3 of MeOH was added at r.t.for 10 min, and the resultant solution was stirred for 1 h. The mixture was concentrated in vacuo. Unreacted diamine was removed by diethyl ether (30 cm3 x 2).The residue was dissolved in DCM (20 cm3), treated with 2 N NaOH solution (15cm3). The product in the organic layer was washed with of brine (20 cm3) dried overanhydrous MgSO4, and concentrated in vacuo to yield a mono-Boc product 15 as awhite solid, (0.68 g, 3.18 mmol, 72%). Mp 109-112 °C; [α]D25 = -1.2, (c 0.1, CHCl3);νmax(neat) 2929, 1691, 1545, 1317, 1240, 1040, 1016, 965, 850 and 759 cm-1; δH (400MHz, CDCl3) 4.46 (1H, br s, NH), 3.14-3.12 (1H, m, CH), 2.32 (1H, td, J 5.6, 10.1,CH), 2.04-1.98 (2H, m, CH2), 1.72-1.69 (2H, m, CH2), 1.45 (9H, s, t-butyl), 1.37 (2H,br s, NH2), 1.29-1.07 (4H, m, 2 x CH2); δC (100 MHz,CDCl3) 156.49 (C=O), 57.69(C), 55.63 (CH), 55.01, (CH), 35.20 (CH2), 32.86 (CH2), 28.36 (3 x CH3), 25.15(CH2), 25.04 (CH2). m/z (ESI) 215.2 [M+H]+. HRMS found (ESI) 215.1751(C11H23N2O2 [M+H]+ requires 215.1754, error= 1.6 ppm). |
| 71% |
With hydrogenchloride In methanol; water at 20℃; for 2h; Inert atmosphere; |
|
| 71% |
Stage #1: (1R,2R)-1,2-diaminocyclohexane With hydrogenchloride In methanol; water at 0 - 20℃;
Stage #2: di-<i>tert</i>-butyl dicarbonate In methanol; water at 20℃; for 4h; |
|
| 70% |
Stage #1: (1R,2R)-1,2-diaminocyclohexane With hydrogenchloride In methanol for 2h;
Stage #2: di-<i>tert</i>-butyl dicarbonate In methanol; water for 3h; |
2.3.1 trans-(1R,2R)-1-Boc-amino-2-aminocyclohexane:
The 800 mL of methanol containing 16.53 g (0.453 mol) ofdry HCl was added dropwise to the solution of 51.78 g (0.453mol) of trans-(1R,2R)-()-1,2-diaminocyclohexane in 80 mLof methanol for 2 h. After adding 50 mL of water, 98.97 g(0.453 mol) of di-t-butyl dicarbonate was added by portions for1 h. The mixture was stirred for 2 h, and then methanol wasremoved. The residue was rinsed with 900 mL of ether and theinsoluble matter was filtered off and dried. The crude productwas dissolved in 800 mL of water and then extracted with amixture of 500 mL of CH2Cl2 and 340 mL of 2 M NaOH. Theorganic layer was retained and treated with MgSO4 and the solvent evaporated. Recrystallization from 900 mL of ligroingave 67.87 g (70%) of trans-(1R,2R)-1-Boc-amino-2-aminocyclohexane. IR (KBr, cm1): 1695 (νC=O urethane), 1554(δN-H amide II). |
| 69.5% |
In dichloromethane Ambient temperature; |
|
|
Stage #1: (1R,2R)-1,2-diaminocyclohexane With hydrogenchloride In methanol at 0℃;
Stage #2: di-<i>tert</i>-butyl dicarbonate In water at 0℃;
Stage #3: With sodium hydroxide |
|
|
In methanol |
|
|
Stage #1: (1R,2R)-1,2-diaminocyclohexane With methanol; thionyl chloride Cooling with ice;
Stage #2: di-<i>tert</i>-butyl dicarbonate With sodium hydroxide |
SOCl2 (9 mL) was added slowly into 50 mL methanol in ice bath. The newly prepared (1R, 2R)-cyclohexanediamine was slowly added to the methanol solution while stirring, and more white substances would be found in the solution. After filtering and washing with ether, (1R, 2R)-cyclohexanediamine hydrochloride was obtained in 72% yield. The mother liquor could be used for the next cycle. (1R, 2R)-cyclohexanediamine hydrochloride (5.62 g, 0.03 mol) and (Boc)2O (6.55g, 0.03mol) were dissolved in 150 mL methanol in flask. Under the condition of ice bath, NaOH solution (containing NaOH 1.2 g, 0.03 mol) was added slowly, and then the reactant reacted at room temperature and stirred overnight. White solid substance was obtained after filtering. The new prepared white solid substance was placed in a beaker and water (20mL) was added. After filtering out insoluble substance, the remaining liquid was extracted with ether to separate the water layer. Potassium carbonate was slowly added to the water layer until pH value of the solution was 9. After extracting with dichloromethane, drying organic layer over anhydrous MgSO4 and steaming out solvent, the unilateral protected intermediate compound 2 was obtained in 46% yield. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping