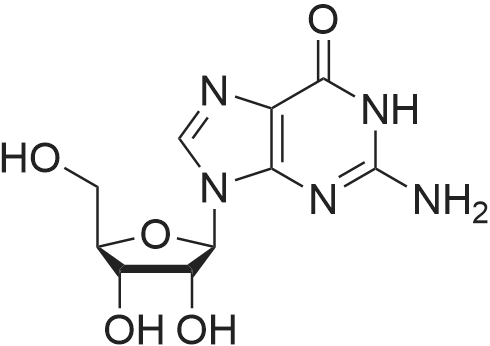
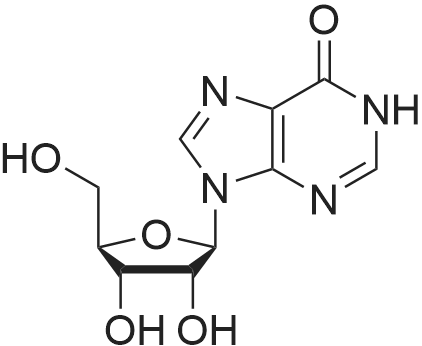
Alternatived Products of [ 146-80-5 ]
Product Details of [ 146-80-5 ]
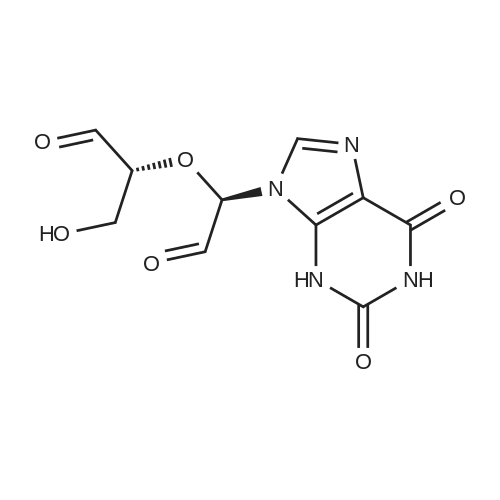
CAS No. : 146-80-5
MDL No. : MFCD00005726
Formula :
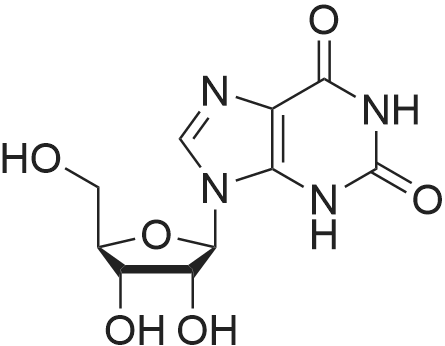
C10 H12 N4 O6
Boiling Point : -
Linear Structure Formula : -
InChI Key : UBORTCNDUKBEOP-UUOKFMHZSA-N
M.W :
284.23
Pubchem ID : 64959
Synonyms :
Xanthine riboside
Chemical Name : 9-((2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-9H-purine-2,6-diol
Calculated chemistry of [ 146-80-5 ]
Physicochemical Properties
Num. heavy atoms : 20
Num. arom. heavy atoms : 9
Fraction Csp3 : 0.5
Num. rotatable bonds : 2
Num. H-bond acceptors : 9.0
Num. H-bond donors : 5.0
Molar Refractivity : 62.32
TPSA : 153.98 Ų
Pharmacokinetics
GI absorption : Low
BBB permeant : No
P-gp substrate : No
CYP1A2 inhibitor : No
CYP2C19 inhibitor : No
CYP2C9 inhibitor : No
CYP2D6 inhibitor : No
CYP3A4 inhibitor : No
Log Kp (skin permeation) : -8.77 cm/s
Lipophilicity
Log Po/w (iLOGP) : 0.79
Log Po/w (XLOGP3) : -1.03
Log Po/w (WLOGP) : -2.48
Log Po/w (MLOGP) : -2.82
Log Po/w (SILICOS-IT) : -2.6
Consensus Log Po/w : -1.63
Druglikeness
Lipinski : 0.0
Ghose : None
Veber : 1.0
Egan : 1.0
Muegge : 1.0
Bioavailability Score : 0.55
Water Solubility
Log S (ESOL) : -1.15
Solubility : 19.9 mg/ml ; 0.0701 mol/l
Class : Very soluble
Log S (Ali) : -1.72
Solubility : 5.47 mg/ml ; 0.0192 mol/l
Class : Very soluble
Log S (SILICOS-IT) : 1.21
Solubility : 4560.0 mg/ml ; 16.0 mol/l
Class : Soluble
Medicinal Chemistry
PAINS : 0.0 alert
Brenk : 0.0 alert
Leadlikeness : 0.0
Synthetic accessibility : 3.92
Application In Synthesis of [ 146-80-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
Downstream synthetic route of [ 146-80-5 ]
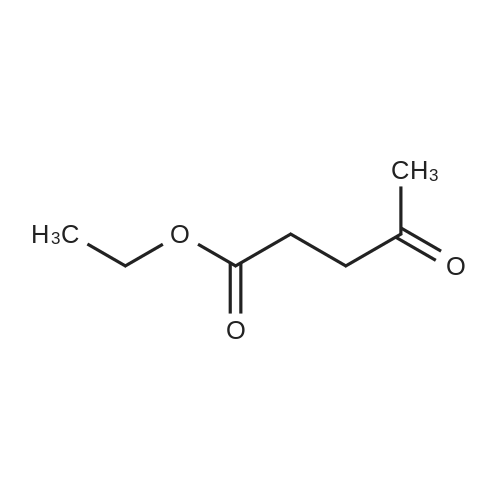
1
[ 539-88-8 ]
[ 122-51-0 ]
[ 146-80-5 ]
[ 77092-71-8 ]
Yield Reaction Conditions Operation in experiment
46.5%
With hydrogenchloride In 1,4-dioxane; N,N-dimethyl-formamide for 24h; Ambient temperature;
2
[ 2485-03-2 ]
[ 146-80-5 ]
[ 58-08-2 ]
Yield Reaction Conditions Operation in experiment
In methanol at 220℃; Yield given;
3
[ 73333-47-8 ]
[ 146-80-5 ]
[ 86013-99-2 ]
Yield Reaction Conditions Operation in experiment
55%
With 2,4,6-trimethyl-pyridine In methanol for 2.5h; Heating;
4
[ 146-80-5 ]
[ 999-97-3 ]
[ 86187-41-9 ]
Yield Reaction Conditions Operation in experiment
66%
With ammonium sulfate 1) RT, 3h, 2) 160 deg C, 9h;
5
[ 146-80-5 ]
[ 132194-28-6 ]
Yield Reaction Conditions Operation in experiment
5.3%
With 2',3'-dideoxyuridine at 50℃; for 4h; Escherichia coli JA-300 cells, pH = 7.0;
6
[ 146-80-5 ]
[ 88010-99-5 ]
Yield Reaction Conditions Operation in experiment
92%
With sodium periodate In water 1.) 30 min 0 deg C, 2.) ambient temperature, 8 h;
7
[ 146-80-5 ]
9-((2R,3R,4S,5R)-3,4-Dihydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-3,9-dihydro-purine-2,6-dione
[ No CAS ]
Yield Reaction Conditions Operation in experiment
With sodium chloride In water at 20℃; Irradiation;
8
[ 146-80-5 ]
[ 69-89-6 ]
9
[ 4016-63-1 ]
[ 146-80-5 ]
8-(8-guanosyl)xanthosine
[ No CAS ]
10
[ 118-00-3 ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
81%
With oxygen; nitrogen(II) oxide In dimethyl sulfoxide for 1h; Ambient temperature;
11
[ 146-80-5 ]
(2S,3S,4R,5R)-5-(2,6-Dioxo-1,2,3,6-tetrahydro-purin-9-yl)-3,4-dihydroxy-tetrahydro-furan-2-carboxylic acid
[ No CAS ]
Yield Reaction Conditions Operation in experiment
With nucleoside oxidase from S. maltophilia In phosphate buffer at 25℃;
Reference:
[1]Mahmoudian, Mahmoud; Rudd, Brian A.M.; Cox, Brian; Drake, Chris S.; Hall, Richard M.; Stead, Paul; Dawson, Michael J.; Chandler, Malcolm; Livermore, David G.; Turner, Nicholas J.; Jenkins, Gareth
[Tetrahedron, 1998, vol. 54, # 28, p. 8171 - 8182]
Yield Reaction Conditions Operation in experiment
With acetic acid; sodium nitrite
13
N1 ,N3 -di(tert-butoxycarbonyl)-9-(5-O-tert-butyldimethylsilyl-2,3-O-isopropylidene-β-D-ribofuranosyl)xanthine
[ No CAS ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
97%
With water; trifluoroacetic acid at 0℃; for 2h;
14
[ 118-00-3 ]
[ 80394-72-5 ]
[ 13276-43-2 ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
With sodium acetate; sodium chloride; sodium nitrite In water at 37℃; for 2h;
15
C10 H11 N6 O6 (1-)
[ No CAS ]
[ 80394-72-5 ]
[ 13276-43-2 ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
With phosphate buffer; sodium chloride In water at 37℃; for 1h;
16
[ 146-80-5 ]
[ 77-76-9 ]
[ 4137-57-9 ]
Yield Reaction Conditions Operation in experiment
With toluene-4-sulfonic acid In acetone
Reference:
[1]Chun, Byoung-Kwon; Wang, Peiyuan; Hassan, Abdalla; Du, Jinfa; Tharnish, Phillip M.; Stuyver, Lieven J.; Otto, Michael J.; Schinazi, Raymond F.; Watanabe, Kyoichi A.
[Tetrahedron Letters, 2005, vol. 46, # 16, p. 2825 - 2827]
17
[ 18162-48-6 ]
[ 146-80-5 ]
2',3',5'-tri-O-(tert-butyldimethylsilyl)xanthosine
[ No CAS ]
Yield Reaction Conditions Operation in experiment
63%
With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 48h;
Reference:
[1]Hupp, Thomas; Sturm, Christian; Janke, Eline M. Basilio; Cabre, Montserrat Perez; Weisz, Klaus; Engels, Bernd
[Journal of Physical Chemistry A, 2005, vol. 109, # 8, p. 1703 - 1712]
18
[ 146-80-5 ]
[ 10380-93-5 ]
Yield Reaction Conditions Operation in experiment
86%
With triphenylphosphine; diethylazodicarboxylate In N,N-dimethyl-formamide at 20℃; for 12h;
52%
With di-isopropyl azodicarboxylate; triphenylphosphine In N,N-dimethyl-formamide at 20℃; for 20.5h;
Multi-step reaction with 3 steps
1: TsOH / acetone
2: Ph3 P; DEAD / dimethylformamide
3: aq. HCl
Multi-step reaction with 2 steps
1: (i) NaHCO3 , DMF, (ii) /BRN= 1074863/
2: NH4 OH
Reference:
[1]Chen, Grace Shiahuy; Chen, Chien-Shu; Chien, Tun-Cheng; Yeh, Jun-Yen; Kuo, Chia-Chi; Talekar, Rahul Subhash; Chern, Ji-Wang
[Nucleosides, nucleotides and nucleic acids, 2004, vol. 23, # 1-2, p. 347 - 359]
[2]Capon, Robert J.; Trotter, Nicholas S.
[Journal of Natural Products, 2005, vol. 68, # 11, p. 1689 - 1691]
[3]Chun, Byoung-Kwon; Wang, Peiyuan; Hassan, Abdalla; Du, Jinfa; Tharnish, Phillip M.; Stuyver, Lieven J.; Otto, Michael J.; Schinazi, Raymond F.; Watanabe, Kyoichi A.
[Tetrahedron Letters, 2005, vol. 46, # 16, p. 2825 - 2827]
[4]Hampton,A.; Nichol,A.W.
[Journal of Organic Chemistry, 1967, vol. 32, p. 1688 - 1691]
19
[ 108-24-7 ]
[ 146-80-5 ]
2',3',5'-O-triacetylxanthosine
[ No CAS ]
Yield Reaction Conditions Operation in experiment
89%
at 100℃; for 1.5h;
20
[ 108-24-7 ]
[ 146-80-5 ]
[ 61444-45-9 ]
Yield Reaction Conditions Operation in experiment
81%
With pyridine at 50℃; for 12h;
21
[ 146-80-5 ]
3,5-dioxo-4,5,6,7-tetrahydro-3<i>H</i>-8-oxa-2,4,5a,9a-tetraaza-benzo[<i>cd</i>]azulene-7,9-dicarbaldehyde
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: 86 percent / triphenylphosphine; diethyl azodicarboxylate / dimethylformamide / 12 h / 20 °C
2: sodium periodate / H2 O / 1 h / 20 °C
Reference:
[1]Chen, Grace Shiahuy; Chen, Chien-Shu; Chien, Tun-Cheng; Yeh, Jun-Yen; Kuo, Chia-Chi; Talekar, Rahul Subhash; Chern, Ji-Wang
[Nucleosides, nucleotides and nucleic acids, 2004, vol. 23, # 1-2, p. 347 - 359]
22
[ 146-80-5 ]
N3 ,5'-cyclo-2',3'-secoxanthosine
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 3 steps
1: 86 percent / triphenylphosphine; diethyl azodicarboxylate / dimethylformamide / 12 h / 20 °C
2: sodium periodate / H2 O / 1 h / 20 °C
3: 0.11 g / sodium borohydride / H2 O / 2 h / 20 °C
Reference:
[1]Chen, Grace Shiahuy; Chen, Chien-Shu; Chien, Tun-Cheng; Yeh, Jun-Yen; Kuo, Chia-Chi; Talekar, Rahul Subhash; Chern, Ji-Wang
[Nucleosides, nucleotides and nucleic acids, 2004, vol. 23, # 1-2, p. 347 - 359]
23
[ 146-80-5 ]
[ 17044-78-9 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: TsOH / acetone
2: Ph3 P; DEAD / dimethylformamide
Reference:
[1]Chun, Byoung-Kwon; Wang, Peiyuan; Hassan, Abdalla; Du, Jinfa; Tharnish, Phillip M.; Stuyver, Lieven J.; Otto, Michael J.; Schinazi, Raymond F.; Watanabe, Kyoichi A.
[Tetrahedron Letters, 2005, vol. 46, # 16, p. 2825 - 2827]
24
[ 197437-76-6 ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: 16 percent / DMAP; Et3 N / 1,2-dichloro-ethane / 0.5 h / 75 °C
2: 97 percent / TFA; H2 O / 2 h / 0 °C
25
[ 146-80-5 ]
[ 77092-70-7 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: 46.5 percent / hydrogen chloride / dioxane; dimethylformamide / 24 h / Ambient temperature
2: 68.7 percent / M sodium hydroxide / ethanol; H2 O / 0.5 h / Ambient temperature
26
[ 146-80-5 ]
9-(3-Amino-3-deoxy-β-D-galactopyranosyl)xanthine acetate
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 3 steps
1: 92 percent / NaIO4 / H2 O / 1.) 30 min 0 deg C, 2.) ambient temperature, 8 h
2: 20 percent / Sodium methoxide / methanol; H2 O / 8 h / 0 - 2 °C
3: 74 percent / H2 / 10 percent Pd/C / H2 O; methanol / 5 h
27
[ 146-80-5 ]
[ 88011-02-3 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: 92 percent / NaIO4 / H2 O / 1.) 30 min 0 deg C, 2.) ambient temperature, 8 h
2: 15 percent / Sodium methoxide / methanol; H2 O / 8 h / 0 - 2 °C
28
[ 146-80-5 ]
[ 88011-00-1 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: 92 percent / NaIO4 / H2 O / 1.) 30 min 0 deg C, 2.) ambient temperature, 8 h
2: 20 percent / Sodium methoxide / methanol; H2 O / 8 h / 0 - 2 °C
29
[ 743369-26-8 ]
[ 146-80-5 ]
[ 133986-29-5 ]
Yield Reaction Conditions Operation in experiment
With H2 O In water stirred for 3 h in warm water;; filtered; crystd. at room temp.; elem. anal.;;
30
nickel(II) nitrate hexahydrate
[ No CAS ]
[ 146-80-5 ]
[ 133986-30-8 ]
Yield Reaction Conditions Operation in experiment
With H2 O In ammonia aq. ammonia=NH3; elem. anal.;;
31
aqueous cadmium chloride
[ No CAS ]
[ 146-80-5 ]
[ 133986-31-9 ]
Yield Reaction Conditions Operation in experiment
With H2 O; NaOH In water byproducts: Cd(OH)2; NaOH added slowly with stirring to xanthosine in water; CdCl2*2.5 H2O was added;; filtered; elem. anal.;;
32
manganese(II)carbonate
[ No CAS ]
[ 146-80-5 ]
[ 133986-23-9 ]
Yield Reaction Conditions Operation in experiment
With H2 O In water stirring at 70°C for 0.5 h;; filtered; cooled; crystn. by slow evapn. in air; elem. anal.;;
33
[ 719261-06-0 ]
[ 146-80-5 ]
[ 133986-30-8 ]
Yield Reaction Conditions Operation in experiment
With H2 O In ammonia aq. ammonia=NH3;
34
[ 146-80-5 ]
[ 759403-02-6 ]
[ 133986-25-1 ]
Yield Reaction Conditions Operation in experiment
With H2 O In water stirring at 70°C for 5 h;; elem. anal.;;
35
copper(II) carbonate
[ No CAS ]
[ 146-80-5 ]
[ 133986-28-4 ]
Yield Reaction Conditions Operation in experiment
With H2 O In water 70°C; 3 h;; filtered warm; evapd.; elem. anal.;;
36
potassium chromate
[ No CAS ]
[ 146-80-5 ]
{CrO(C4 H4 O(CH2 OH)(O)2C5 HN4 (OH)2)2}
[ No CAS ]
Yield Reaction Conditions Operation in experiment
With formaldehyd In water; dimethyl sulfoxide standing in a closed flask at 25°C for 8 days; monitoring by EPR;
37
[ 146-80-5 ]
palladium dichloride
[ No CAS ]
dithiocyanato xanthosine palladium(II)
[ No CAS ]
Yield Reaction Conditions Operation in experiment
With KSCN In water dissoln. of PdCl2 in KCl soln.; addn. to hot xanthosine soln.; addn. of KSCN soln. (in minimum amt. of water); immediate pptn.; filtration; washing (small portions of warm water, ethanol); drying (110°C, 24h); elem. anal.;
38
[ 146-80-5 ]
palladium dichloride
[ No CAS ]
dichloro bis(xanthosine) palladium(II)
[ No CAS ]
Yield Reaction Conditions Operation in experiment
With KCl In water dissoln. of PdCl2 in KCl soln.; addn. to hot xanthosine soln.; leaving to stand at room temp.; cooling; pptn.; filtration, washing (water, ethanol, ether); drying (110°C, 24h); elem. anal.;
39
[ 146-80-5 ]
palladium dichloride
[ No CAS ]
dibromo bis(xanthosine) palladium(II)
[ No CAS ]
Yield Reaction Conditions Operation in experiment
With KBr In water dissoln. of PdCl2 in KBr soln.; addn. to hot xanthosine soln.; leaving to stand at room temp.; cooling; pptn.; filtration, washing (water, ethanol, ether); drying (110°C, 24h); elem. anal.;
40
[ 1184-57-2 ]
[ 146-80-5 ]
(CH3 Hg)2((C5 HN4 O2 )(C4 H4 O)(OH)2(CH2 OH))*H2 O
[ No CAS ]
Yield Reaction Conditions Operation in experiment
85%
In water dissolving xanthosine in a boiling mixt. of CH3HgOH and water, stirringfor 1 h, cooling; after 3 days filtn., washing with ethanol, drying at room temp. in vacuo, elem. anal.;
41
(hydrotris(3-p-cumenylmethylpyrazolyl)borate)Zn(OH)
[ No CAS ]
[ 146-80-5 ]
(tris(3-cumenyl-5-methylpyrazolyl)borate)Zn(xanthosinate)
[ No CAS ]
Yield Reaction Conditions Operation in experiment
61%
In methanol; dichloromethane addn. of 1 equiv. ligand (in MeOH) to Zn-complex (in CH2Cl2), stirring for 2 h; solvent removal (vac.), dissoln. in boiling cyclohexane, crystn. (3 d), collection (filtration), drying (vac.); elem. anal.;
42
K[Pd(guanosine)Cl3 ]
[ No CAS ]
[ 146-80-5 ]
cis-(guanosine)(xanthosine)palladium(II) dichloride
[ No CAS ]
Yield Reaction Conditions Operation in experiment
65%
In hydrogenchloride equimolar amts., stirring in 0.5 M HCl for 2 h (pptn.); filtration, washing (0.5 M HCl, Me2CO, Et2O), drying (110°C, vac.); elem. anal.;
43
[ 146-80-5 ]
[ 58-63-9 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: inosine-uridine nucleoside hydrolase from Escherichia coli / aq. buffer / 32 °C / pH 7.2 / Enzymatic reaction
2: diothiothreitol; inosine-uridine nucleoside hydrolase from Escherichia coli / aq. buffer / 37 °C / pH 7.2 / Enzymatic reaction
Reference:
[1]Arivett, Brock; Farone, Mary; Masiragani, Ranjith; Burden, Andrew; Judge, Shelby; Osinloye, Adedoyin; Minici, Claudia; Degano, Massimo; Robinson, Matthew; Kline, Paul
[Biochimica et Biophysica Acta - Proteins and Proteomics, 2014, vol. 1844, # 3, p. 656 - 662]
44
[ 146-80-5 ]
XMP
[ No CAS ]
Yield Reaction Conditions Operation in experiment
81%
Stage #1: xanthosine With N,N,N',N'-tetramethyl-1,8-diaminonaphthalene; trichlorophosphate at 20℃; for 0.25h; Flow reactor; Green chemistry;
Stage #2: With water at 20℃; Flow reactor; Green chemistry; chemoselective reaction;
Reference:
[1]Zhu, Chenjie; Tang, Chenglun; Cao, Zhi; He, Wei; Chen, Yong; Chen, Xiaochun; Guo, Kai; Ying, Hanjie
[Organic Process Research and Development, 2014, vol. 18, # 11, p. 1575 - 1581]
45
[ 2466-76-4 ]
[ 146-80-5 ]
[ 61444-45-9 ]
Yield Reaction Conditions Operation in experiment
63%
With sodium hydroxide In water at 20℃; for 4h;
General Nucleoside Acetylation Protocol
General procedure: Nucleoside/nucleotide (2; 100 mM) and N-acetyl imidazole (1a;10 equiv) were dissolved in water (pH 8; adjusted with 4 MNaOH). The solution was incubated at r.t. for 4 h, and NMR spectra were periodically acquired. The product was purified byreverse-phase (C18) flash coumn chromatography (eluted at pH4 with 100 mM NH4HCO2/MeCN = 98:2 to 80:20). The fractions containing 5 were lyophilised to yield a white powder.
46
xanthosine 2-(triethylammoniumphenylphosphate)
[ No CAS ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
In aq. phosphate buffer at 37℃;
47
2',3',5'-O-tris(triisopropylsilyl)-guanosine
[ No CAS ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 7 steps
1.1: diethylazodicarboxylate; triphenylphosphine / tetrahydrofuran; toluene / 48 h / 20 °C / Inert atmosphere
2.1: sodium nitrite; acetic acid / water; acetone; dichloromethane / 48 h / 0 °C
2.2: 48 h / 20 °C
3.1: dimethyl(cyanomethyl)ammonium trifluoromethanesulfonate / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
4.1: rac-camphorsulfonyloxaziridine / tetrahydrofuran; acetonitrile / 10 h / 20 °C / Inert atmosphere
5.1: 1,8-diazabicyclo[5.4.0]undec-7-ene; nitromethane / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
5.2: 3407 / pH 7
6.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 2 h / 20 °C / Inert atmosphere
7.1: aq. phosphate buffer / 37 °C / pH 4.8
48
[ 72409-24-6 ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 7 steps
1.1: diethylazodicarboxylate; triphenylphosphine / tetrahydrofuran; toluene / 48 h / 20 °C / Inert atmosphere
2.1: sodium nitrite; acetic acid / water; acetone / 0 °C
2.2: 20 °C
3.1: dimethyl(cyanomethyl)ammonium trifluoromethanesulfonate / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
4.1: rac-camphorsulfonyloxaziridine / tetrahydrofuran; acetonitrile / 21 h / 20 °C / Inert atmosphere
5.1: 1,8-diazabicyclo[5.4.0]undec-7-ene; nitromethane / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
5.2: 3407 / pH 7
6.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere
7.1: aq. phosphate buffer / 37 °C / pH 4.8
49
C45 H69 N6 O11 PSi3
[ No CAS ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 3 steps
1.1: 1,8-diazabicyclo[5.4.0]undec-7-ene; nitromethane / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
1.2: 3407 / pH 7
2.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere
3.1: aq. phosphate buffer / 37 °C / pH 4.8
50
2′,3′,5′-tris-O-(triisopropylsilyl)-6-O-[2-(4-nitrophenyl)ethyl]guanosine
[ No CAS ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 6 steps
1.1: sodium nitrite; acetic acid / water; acetone; dichloromethane / 48 h / 0 °C
1.2: 48 h / 20 °C
2.1: dimethyl(cyanomethyl)ammonium trifluoromethanesulfonate / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
3.1: rac-camphorsulfonyloxaziridine / tetrahydrofuran; acetonitrile / 10 h / 20 °C / Inert atmosphere
4.1: 1,8-diazabicyclo[5.4.0]undec-7-ene; nitromethane / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
4.2: 3407 / pH 7
5.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 2 h / 20 °C / Inert atmosphere
6.1: aq. phosphate buffer / 37 °C / pH 4.8
51
C45 H69 N6 O10 PSi3
[ No CAS ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 4 steps
1.1: rac-camphorsulfonyloxaziridine / tetrahydrofuran; acetonitrile / 21 h / 20 °C / Inert atmosphere
2.1: 1,8-diazabicyclo[5.4.0]undec-7-ene; nitromethane / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
2.2: 3407 / pH 7
3.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere
4.1: aq. phosphate buffer / 37 °C / pH 4.8
52
[ 1203555-53-6 ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 6 steps
1.1: sodium nitrite; acetic acid / water; acetone / 0 °C
1.2: 20 °C
2.1: dimethyl(cyanomethyl)ammonium trifluoromethanesulfonate / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
3.1: rac-camphorsulfonyloxaziridine / tetrahydrofuran; acetonitrile / 21 h / 20 °C / Inert atmosphere
4.1: 1,8-diazabicyclo[5.4.0]undec-7-ene; nitromethane / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
4.2: 3407 / pH 7
5.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere
6.1: aq. phosphate buffer / 37 °C / pH 4.8
53
2′,3′,5′-tris-O-(triisopropylsilyl)-6-O-[2-(4-nitrophenyl)ethyl]xanthosine
[ No CAS ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 5 steps
1.1: dimethyl(cyanomethyl)ammonium trifluoromethanesulfonate / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
2.1: rac-camphorsulfonyloxaziridine / tetrahydrofuran; acetonitrile / 10 h / 20 °C / Inert atmosphere
3.1: 1,8-diazabicyclo[5.4.0]undec-7-ene; nitromethane / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
3.2: 3407 / pH 7
4.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 2 h / 20 °C / Inert atmosphere
5.1: aq. phosphate buffer / 37 °C / pH 4.8
54
2′,3′,5′-tris-O-(tert-butyldimethylsilyl)-6-O-[2-(4-nitrophenyl)ethyl]xanthosine
[ No CAS ]
[ 146-80-5 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 5 steps
1.1: dimethyl(cyanomethyl)ammonium trifluoromethanesulfonate / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
2.1: rac-camphorsulfonyloxaziridine / tetrahydrofuran; acetonitrile / 21 h / 20 °C / Inert atmosphere
3.1: 1,8-diazabicyclo[5.4.0]undec-7-ene; nitromethane / tetrahydrofuran; acetonitrile / 1 h / 20 °C / Inert atmosphere
3.2: 3407 / pH 7
4.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere
5.1: aq. phosphate buffer / 37 °C / pH 4.8

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping