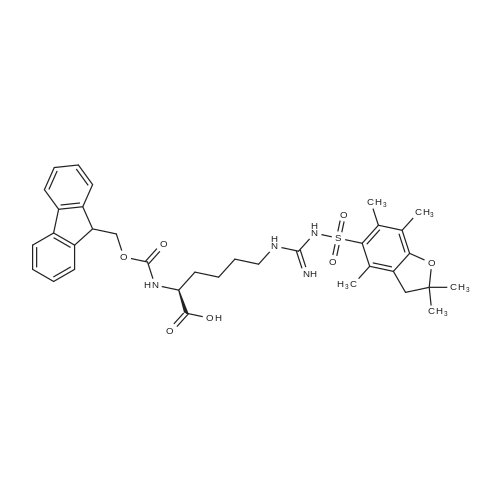
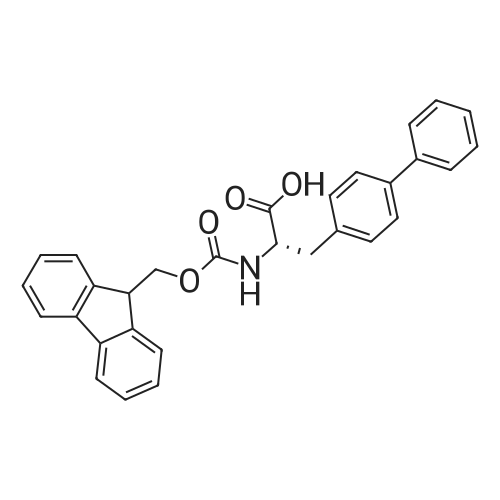
Alternatived Products of [ 1430938-32-1 ]
Product Details of [ 1430938-32-1 ]
CAS No. : 1430938-32-1
MDL No. : MFCD24395695
Formula :
C12 H21 NO4
Boiling Point : -
Linear Structure Formula : -
InChI Key : JSGHMGKJNZTKGF-RKDXNWHRSA-N
M.W :
243.30
Pubchem ID : 24720881
Synonyms :
Calculated chemistry of [ 1430938-32-1 ]
Physicochemical Properties
Num. heavy atoms : 17
Num. arom. heavy atoms : 0
Fraction Csp3 : 0.83
Num. rotatable bonds : 5
Num. H-bond acceptors : 4.0
Num. H-bond donors : 2.0
Molar Refractivity : 63.97
TPSA : 75.63 Ų
Pharmacokinetics
GI absorption : High
BBB permeant : Yes
P-gp substrate : No
CYP1A2 inhibitor : No
CYP2C19 inhibitor : No
CYP2C9 inhibitor : No
CYP2D6 inhibitor : No
CYP3A4 inhibitor : No
Log Kp (skin permeation) : -6.56 cm/s
Lipophilicity
Log Po/w (iLOGP) : 2.19
Log Po/w (XLOGP3) : 1.73
Log Po/w (WLOGP) : 2.15
Log Po/w (MLOGP) : 1.31
Log Po/w (SILICOS-IT) : 0.83
Consensus Log Po/w : 1.64
Druglikeness
Lipinski : 0.0
Ghose : None
Veber : 0.0
Egan : 0.0
Muegge : 0.0
Bioavailability Score : 0.56
Water Solubility
Log S (ESOL) : -2.11
Solubility : 1.9 mg/ml ; 0.00779 mol/l
Class : Soluble
Log S (Ali) : -2.93
Solubility : 0.283 mg/ml ; 0.00116 mol/l
Class : Soluble
Log S (SILICOS-IT) : -1.42
Solubility : 9.29 mg/ml ; 0.0382 mol/l
Class : Soluble
Medicinal Chemistry
PAINS : 0.0 alert
Brenk : 0.0 alert
Leadlikeness : 1.0
Synthetic accessibility : 3.07
Safety of [ 1430938-32-1 ]
Application In Synthesis of [ 1430938-32-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
Downstream synthetic route of [ 1430938-32-1 ]
1
[ 1430938-32-1 ]
C20 H20 N4 O2
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 3 steps
1: triethylamine; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dichloromethane / 20 °C
2: trifluoroacetic acid / dichloromethane / 0.5 h
3: triethylamine; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dichloromethane / 20 °C
Reference:
[1]Zhou, Hao; Topiol, Sidney W.; Grenon, Michel; Jimenez, Hermogenes N.; Uberti, Michelle A.; Smith, Daniel G.; Brodbeck, Robbin M.; Chandrasena, Gamini; Pedersen, Henrik; Madsen, Jens Christian; Doller, Darío; Li, Guiying
[Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 5, p. 1398 - 1406]
2
[ 1430938-32-1 ]
C19 H20 ClN3 O2
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 3 steps
1: triethylamine; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dichloromethane / 20 °C
2: trifluoroacetic acid / dichloromethane / 0.5 h
3: triethylamine; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dichloromethane / 20 °C
Reference:
[1]Zhou, Hao; Topiol, Sidney W.; Grenon, Michel; Jimenez, Hermogenes N.; Uberti, Michelle A.; Smith, Daniel G.; Brodbeck, Robbin M.; Chandrasena, Gamini; Pedersen, Henrik; Madsen, Jens Christian; Doller, Darío; Li, Guiying
[Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 5, p. 1398 - 1406]
3
[ 1430938-32-1 ]
C19 H20 ClN3 O2
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 3 steps
1: triethylamine; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dichloromethane / 20 °C
2: trifluoroacetic acid / dichloromethane / 0.5 h / 20 °C
3: triethylamine; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dichloromethane / 20 °C
Reference:
[1]Zhou, Hao; Topiol, Sidney W.; Grenon, Michel; Jimenez, Hermogenes N.; Uberti, Michelle A.; Smith, Daniel G.; Brodbeck, Robbin M.; Chandrasena, Gamini; Pedersen, Henrik; Madsen, Jens Christian; Doller, Darío; Li, Guiying
[Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 5, p. 1398 - 1406]
4
[ 1430938-32-1 ]
C12 H17 N3 O
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: triethylamine; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dichloromethane / 20 °C
2: trifluoroacetic acid / dichloromethane / 0.5 h
Reference:
[1]Zhou, Hao; Topiol, Sidney W.; Grenon, Michel; Jimenez, Hermogenes N.; Uberti, Michelle A.; Smith, Daniel G.; Brodbeck, Robbin M.; Chandrasena, Gamini; Pedersen, Henrik; Madsen, Jens Christian; Doller, Darío; Li, Guiying
[Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 5, p. 1398 - 1406]
5
[ 1430938-32-1 ]
C13 H17 ClN2 O
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: triethylamine; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate / dichloromethane / 20 °C
2: trifluoroacetic acid / dichloromethane / 0.5 h / 20 °C
Reference:
[1]Zhou, Hao; Topiol, Sidney W.; Grenon, Michel; Jimenez, Hermogenes N.; Uberti, Michelle A.; Smith, Daniel G.; Brodbeck, Robbin M.; Chandrasena, Gamini; Pedersen, Henrik; Madsen, Jens Christian; Doller, Darío; Li, Guiying
[Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 5, p. 1398 - 1406]
6
[ 1430938-32-1 ]
[ 75-65-0 ]
trans-1,3-cyclohexanedi(tert-butoxycarbonyl)amine
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Stage #1: (1R,3R)-3-((tert-butoxycarbonyl)amino)cyclohexanecarboxylic acid With diphenyl phosphoryl azide; triethylamine In toluene for 1h; Reflux;
Stage #2: <i>tert</i>-butyl alcohol In toluene for 12h; Reflux;
Reference:
[1]Zhou, Hao; Topiol, Sidney W.; Grenon, Michel; Jimenez, Hermogenes N.; Uberti, Michelle A.; Smith, Daniel G.; Brodbeck, Robbin M.; Chandrasena, Gamini; Pedersen, Henrik; Madsen, Jens Christian; Doller, Darío; Li, Guiying
[Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 5, p. 1398 - 1406]
7
[ 504-29-0 ]
[ 1430938-32-1 ]
C17 H25 N3 O3
[ No CAS ]
Yield Reaction Conditions Operation in experiment
With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane at 20℃;
Reference:
[1]Zhou, Hao; Topiol, Sidney W.; Grenon, Michel; Jimenez, Hermogenes N.; Uberti, Michelle A.; Smith, Daniel G.; Brodbeck, Robbin M.; Chandrasena, Gamini; Pedersen, Henrik; Madsen, Jens Christian; Doller, Darío; Li, Guiying
[Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 5, p. 1398 - 1406]
8
[ 1430938-32-1 ]
[ 108-42-9 ]
C18 H25 ClN2 O3
[ No CAS ]
Yield Reaction Conditions Operation in experiment
With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane at 20℃;
Reference:
[1]Zhou, Hao; Topiol, Sidney W.; Grenon, Michel; Jimenez, Hermogenes N.; Uberti, Michelle A.; Smith, Daniel G.; Brodbeck, Robbin M.; Chandrasena, Gamini; Pedersen, Henrik; Madsen, Jens Christian; Doller, Darío; Li, Guiying
[Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 5, p. 1398 - 1406]
9
C25 H33 NO5 S
[ No CAS ]
[ 1430938-32-1 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: magnesium / methanol / 1 h / 20 °C / Sonication
2: ruthenium(III) trichloride hydrate; sodium periodate / water; acetonitrile; ethyl acetate / 1.5 h / 20 °C
10
(1R,3R)-3-(4-methoxyphenyl)cyclohexyl-tert-butylcarbamate
[ No CAS ]
[ 1430938-32-1 ]
Yield Reaction Conditions Operation in experiment
60%
With sodium periodate; ruthenium(III) trichloride hydrate In water; ethyl acetate; acetonitrile at 20℃; for 1.5h;
11
[ 24424-99-5 ]
[ 1430938-32-1 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 3 steps
1: dmap / acetonitrile / 3 h / 20 °C
2: magnesium / methanol / 1 h / 20 °C / Sonication
3: ruthenium(III) trichloride hydrate; sodium periodate / water; acetonitrile; ethyl acetate / 1.5 h / 20 °C
12
N-[(1R,3R)-3-(4-methoxyphenyl)cyclohexyl]-4-methylbenzenesulfonamide
[ No CAS ]
[ 1430938-32-1 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 3 steps
1: dmap / acetonitrile / 3 h / 20 °C
2: magnesium / methanol / 1 h / 20 °C / Sonication
3: ruthenium(III) trichloride hydrate; sodium periodate / water; acetonitrile; ethyl acetate / 1.5 h / 20 °C
13
[ 1430938-32-1 ]
[ 76-05-1 ]
(1R,3R)-3-aminocyclohexanecarboxylic acid trifluoroacetate
[ No CAS ]
Yield Reaction Conditions Operation in experiment
86%
In dichloromethane at 20℃; for 3h;
192.1
Step 1: A mixture of (1R,3R)-3-((tert-butoxycarbonyl)amino)cyclohexanecarboxylic acid in a 1:1 solution ofTFA: CH2Cl2 (6 mL) was stirred at rt for 3 h. The mixture was concentrated under reduced pressure. The residue wastriturated in ethyl ether (50 mL) and the resulting solid was collected by filtration to afford (1R,3R)-3-aminocyclohexanecarboxylicacid trifluoroacetate (253 mg, 86%) as a white solid that did not require further purification.
14
[ 1159680-21-3 ]
[ 1430938-32-1 ]
[ 199110-64-0 ]
N-(9-fluorenylmethyloxycarbonyl)-5-hydroxy-L-tryptophan
[ No CAS ]
(1R,3R)-N-((S)-1-(((S)-3-([1,1'-biphenyl]-4-yl)-1-(((S)-1-amino-6-guanidino-1-oxohexan-2-yl)amino)-1-oxopropan-2-yl)amino)-3-(5-hydroxy-1H-indol-3-yl)-1-oxopropan-2-yl)-3-aminocyclohexane-1-carboxamide
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Stage #1: (S)-2-(((9H-fluoren-9-yl)methoxy)carbonylamino)-6-(3-(2,2,4,6,7-pentamethyl-2,3-dihydrobenzofuran-5-ylsulfonyl)guanidino)hexanoic acid With N-ethyl-N,N-diisopropylamine; ethyl cyanoglyoxylate-2-oxime; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 1h;
Stage #2: With 4-methylpiperidin In N,N-dimethyl-formamide at 20℃;
Stage #3: (1R,3R)-3-((tert-butoxycarbonyl)amino)cyclohexanecarboxylic acid; (S)-3-biphenyl-4-yl-2-(9H-fluoren-9-ylmethoxycarbonylamino)propionic acid; N-(9-fluorenylmethyloxycarbonyl)-5-hydroxy-L-tryptophan Further stages;
Reference:
[1]Baggio, Carlo; Kulinich, Anna; Dennys, Cassandra N.; Rodrigo, Rochelle; Meyer, Kathrin; Ethell, Iryna; Pellecchia, Maurizio
[Journal of Medicinal Chemistry, 2021, vol. 64, # 15, p. 11229 - 11246]
15
[ 1430938-32-1 ]
4-decyl-N'-hydroxybenzimidamide
[ No CAS ]
tert-butyl ((1R,3R)-3-(3-(4-decylphenyl)-1,2,4-oxadiazol-5-yl)cyclohexyl)carbamate
[ No CAS ]
Yield Reaction Conditions Operation in experiment
73%
With N,N,N′,N′-tetramethyl-O-(6-chloro-1H-benzotriazol-1-yl)uronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 100℃; for 6h; Schlenk technique; Inert atmosphere;
Reference:
[1]Fritzemeier, Russell; Foster, Daniel; Peralta, Ashley; Payette, Michael; Kharel, Yugesh; Huang, Tao; Lynch, Kevin R.; Santos, Webster L.
[Journal of Medicinal Chemistry, 2022, vol. 65, # 11, p. 7656 - 7681]
16
[ 1430938-32-1 ]
4-decyl-N'-hydroxybenzimidamide
[ No CAS ]
(1R,3R)-3-(3-(4-decylphenyl)-1,2,4-oxadiazol-5-yl)cyclohexan-1-amine hydrochloride
[ No CAS ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: N-ethyl-N,N-diisopropylamine; N,N,N′,N′-tetramethyl-O-(6-chloro-1H-benzotriazol-1-yl)uronium hexafluorophosphate / N,N-dimethyl-formamide / 6 h / 100 °C / Schlenk technique; Inert atmosphere
2: hydrogenchloride / 1,4-dioxane; dichloromethane / 2 h / 20 °C / Schlenk technique; Inert atmosphere
Reference:
[1]Fritzemeier, Russell; Foster, Daniel; Peralta, Ashley; Payette, Michael; Kharel, Yugesh; Huang, Tao; Lynch, Kevin R.; Santos, Webster L.
[Journal of Medicinal Chemistry, 2022, vol. 65, # 11, p. 7656 - 7681]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping