| 41% |
With palladium (II) [1,1'-bis(diphenylphosphanyl)ferrocene] dichloride; anhydrous potassium acetate In 1,4-dioxane at 80℃; for 16h; Inert atmosphere; |
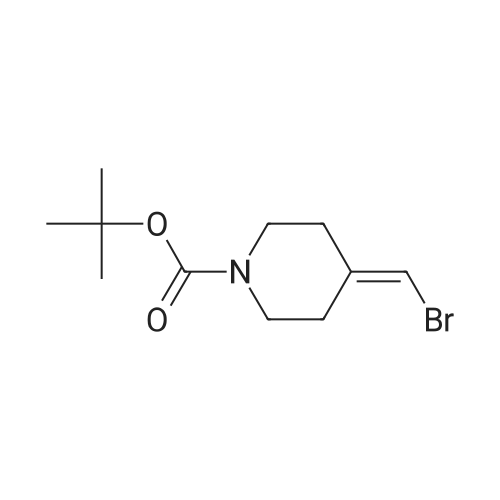
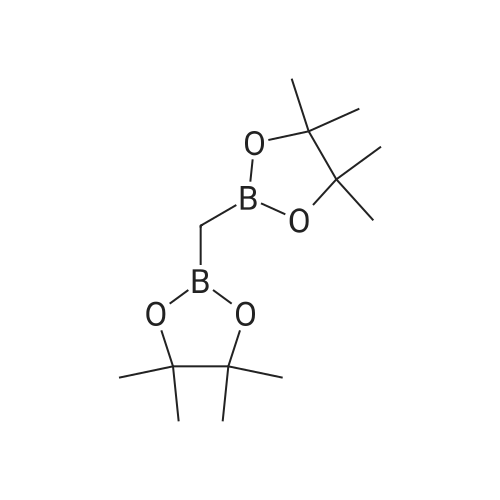
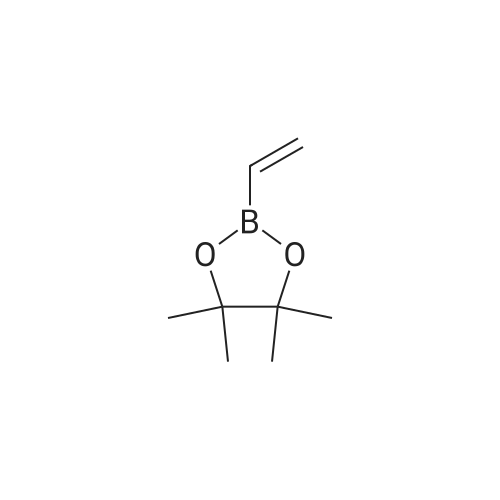
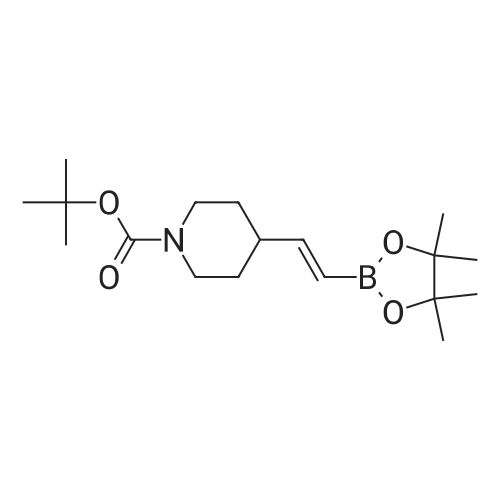
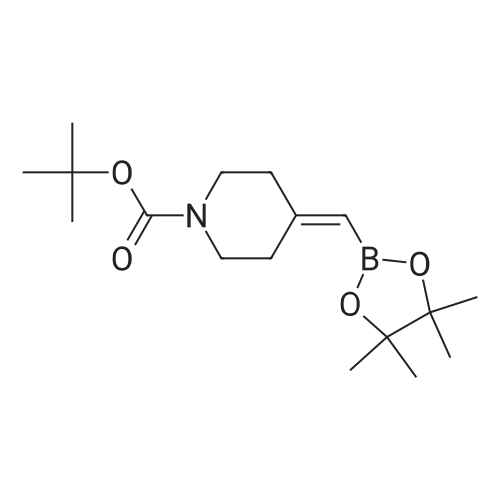
18 tert-butyl 4-[(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)methylidene]piperidine-1-carboxylate
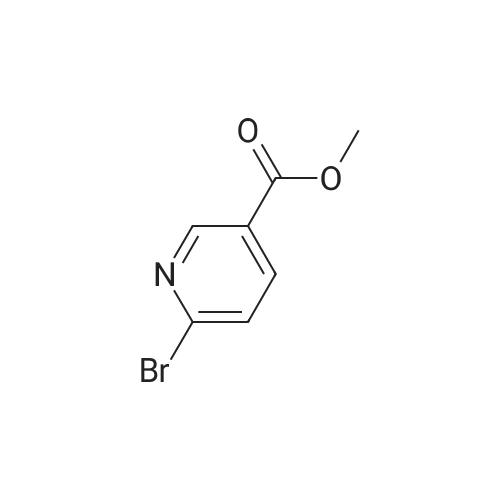
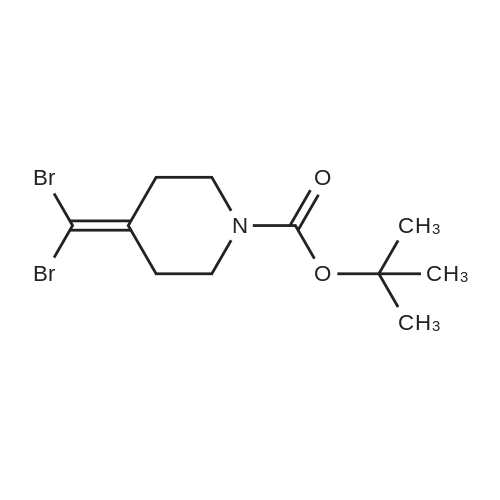
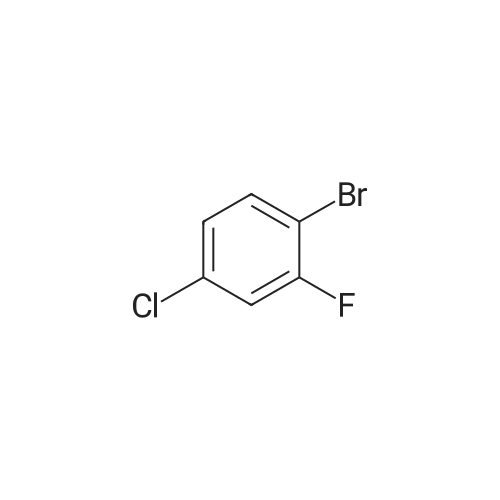
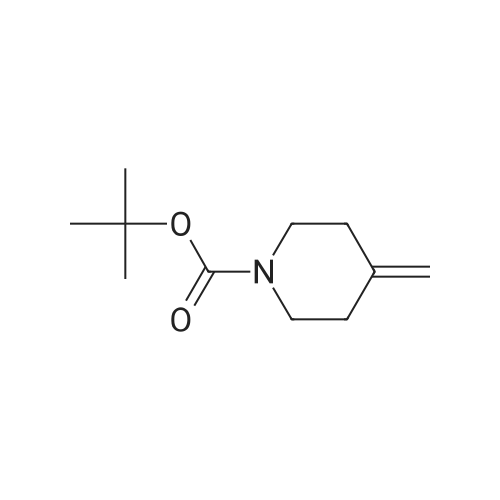
To a stirred solution/mixture of tert-butyl 4-(bromomethylidene)piperidine-1-carboxylate (2.50 g, 9.052 mmol, 1 equiv) and bis(pinacolato)diboron (3.45 g, 13.578 mmol, 1.5 equiv) in 1,4-dioxane were added Pd(dppf)Cl2 (0.66 g, 0.905 mmol, 0.10 equiv) and KOAc (2.67 g, 27.157 mmol, 3 equiv) at room temperature under nitrogen atmosphere. The resulting mixture was stirred for 16 hours at 80 °C under nitrogen atmosphere. The reaction was quenched with Water at room temperature. The resulting mixture was extracted with EtOAc (3 x 30 mL). The combined organic layers were washed with brine (2 x 20 mL), dried over anhydrous Na2SC>4. After filtration, the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography, eluting with petroleum ether/EtOAc (5:1) to afford tert-butyl 4-[(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)methylidene]piperidine-1-carboxylate (1.2 g, 41%) as an off-white solid. |
| 41% |
With palladium (II) [1,1'-bis(diphenylphosphanyl)ferrocene] dichloride; anhydrous potassium acetate In 1,4-dioxane at 80℃; for 16h; Inert atmosphere; |
18 tert-butyl 4-[(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)methylidene]piperidine-1-carboxylate
To a stirred solution/mixture of tert-butyl 4-(bromomethylidene)piperidine-1-carboxylate (2.50 g, 9.052 mmol, 1 equiv) and bis(pinacolato)diboron (3.45 g, 13.578 mmol, 1.5 equiv) in 1,4-dioxane were added Pd(dppf)Cl2 (0.66 g, 0.905 mmol, 0.10 equiv) and KOAc (2.67 g, 27.157 mmol, 3 equiv) at room temperature under nitrogen atmosphere. The resulting mixture was stirred for 16 hours at 80 °C under nitrogen atmosphere. The reaction was quenched with Water at room temperature. The resulting mixture was extracted with EtOAc (3 x 30 mL). The combined organic layers were washed with brine (2 x 20 mL), dried over anhydrous Na2SC>4. After filtration, the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography, eluting with petroleum ether/EtOAc (5:1) to afford tert-butyl 4-[(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)methylidene]piperidine-1-carboxylate (1.2 g, 41%) as an off-white solid. |
| 720 mg |
With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; anhydrous potassium acetate; triphenylphosphine In 1,4-dioxane at 100℃; for 4h; Sealed tube; Inert atmosphere; |
88 Preparation of ferf-butyl 4-[(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl methylenelpiperidine-l - carboxylate
To a re-sealable tube was charged tert-butyl 4-(bromomethylene)piperidine-l-carboxylate (700 mg; 2.51 mmol), potassium acetate (620 mg; 6.27 mmol), bis(pinacolato)diboron (1040 mg; 4.01 mmol) and dioxane (20 mL) at room temperaure. Argon was bubbled in the reaction mixture for 10 minutes and triphenylphosphine (70 mg; 0.25 mmol) and tris(dibenzylideneacetone)dipalladium-chloroform adduct (160 mg; 0.15 mmol) were added. The tube was flushed with argon and sealed. The reaction mixture was then heated to 100 °C and stirred for 4 hours. After cooling to room temperature, the reaction mixture was filtered and the cake was washed with ethyl acetate. The filtrate was finally concentrated to dryness. The crude residue was then purified by column chromatography (silica gel; cyclohexane:ethylacetate; 1 :0 to 4: 1 ; v/v) to afford tert-butyl 4-[(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)methylene]piperidine-l- carboxylate (720 mg) as a light yellow solid. 'H-NMR (400 MHz, CDCI3) δ ppm: 5.17 (s, 1H), 3.42 - 3.48 (m,4H), 2.62 (m, 2H), 2.28 (m, 2H), 1.49 (s, 9H), 1.28 (s, 12H). |
| 720 mg |
With tris-(dibenzylideneacetone)dipalladium(0); anhydrous potassium acetate; triphenylphosphine In 1,4-dioxane at 100℃; for 4h; Sealed tube; Inert atmosphere; |
2.1-b Step 1-b: Preparation of ferf-butyl 4-r(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)methylene1piperidine- 1-carboxylate:
A sealable tube was charged with teri-butyl 4-(bromomethylene)piperidine-l-carboxylate (700 mg; 2.51 mmol), potassium acetate (620 mg; 6.27 mmol), bis(pinacolato)diboron (1040 mg; 4.01 mmol) and dioxane (20 mL) at rt. Argon was bubbled in the reaction mixture for 10 min and triphenylphosphine (70 mg; 0.25 mmol) and Pd2dba3 (160 mg; 0.15 mmol) were added. The tube was flushed with argon and sealed. The reaction mixture was then heated to 100 °C and stirred for 4 h. After cooling to rt, the reaction mixture was filtered and the cake was washed with EA. The filtrate was finally concentrated to dryness. The residue was then purified by column chromatography (silica gel; c-Hex : EA; 1 :0 to 4:1 ; v/v) to afford teri-butyl 4-[(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)methylene]piperidine-l -carboxylate (720 mg) as a light yellow solid. (0713) 'H-NMR (400 MHz, CDCI3) δ ppm: 5.17 (s, 1H), 3.48 - 3.42 (m, 4H), 2.62 (m, 2H), 2.28 (m, 2H), 1.49 (s, 9H), 1.28 (s, 12H). |
| 720 mg |
With tris-(dibenzylideneacetone)dipalladium(0); anhydrous potassium acetate; triphenylphosphine In 1,4-dioxane at 100℃; for 4h; Inert atmosphere; Sealed tube; |
1.1-b Step 1-b: Preparation of ieri-butyl 4-r(4.4.5.5-tetramethyl-l.3.2-dioxaborolan-2-yl)methylene1piperidine- l-carboxylate
A sealable tube was charged with ieri-butyl 4-(bromomethylene)piperidine-l-carboxylate (700 mg; 2.51 mmol), potassium acetate (620 mg; 6.27 mmol), bis(pinacolato)diboron (1 040 mg; 4.01 mmol) and dioxane (20 mL) at rt. Argon was bubbled in the reaction mixture for 10 min and triphenylphosphine (70 mg; 0.25 mmol) and Pd dba3 (160 mg; 0.15 mmol) were added. The tube was flushed with argon and sealed. The reaction mixture was then heated to 100 °C and stirred for 4 h. After cooling to rt, the reaction mixture was filtered and the cake was washed with EA. The filtrate was finally concentrated to dryness. The residue was then purified by column chromatography (silica gel; c-Hex : EA; 1 :0 to 4:1 ; v/v) to afford ieri-butyl 4-[(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)methylene]piperidine-l-carboxylate (720 mg) as a light yellow solid. (0253) ‘H-NMR (400 MHz, CDCI3) d ppm: 5.17 (s, 1H), 3.48 - 3.42 (m, 4H), 2.62 (m, 2H), 2.28 (m, 2H), 1.49 (s, 9H), 1.28 (s, 12H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping