Alternatived Products of [ 139564-41-3 ]
Product Details of [ 139564-41-3 ]
| CAS No. : | 139564-41-3 |
MDL No. : | MFCD19220105 |
| Formula : |
C7H13NO
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | IASLVFGXHJQWAB-LURJTMIESA-N |
| M.W : |
127.18
|
Pubchem ID : | 13082303 |
| Synonyms : |
|
Safety of [ 139564-41-3 ]
Application In Synthesis of [ 139564-41-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 139564-41-3 ]
- 1
-
 [ 139564-39-9 ]
[ 139564-39-9 ]

-
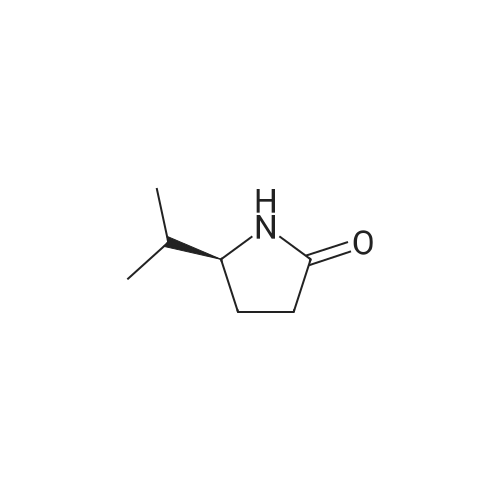 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 81% |
With ammonia; sodium In tetrahydrofuran; ethanol -33 deg C to r.t., 4 h; |
|
- 2
-
 [ 935658-79-0 ]
[ 935658-79-0 ]

-
 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 70% |
Stage #1: (R,E)-dibenzyl 1-(6-ethoxy-2-methyl-6-oxohex-4-en-3-yl)hydrazine-1,2-dicarboxylate With hydrogen In methanol for 12h;
Stage #2: In ethanol at 50℃; for 5h; |
|
- 3
-
 [ 1064654-96-1 ]
[ 1064654-96-1 ]

-
 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1.1: LiBr; DBU / acetonitrile / 0.75 h / 5 °C
2.1: H2 / Raney-Ni / methanol / 12 h / 3102.89 Torr
2.2: 70 percent / ethanol / 5 h / 50 °C |
|
- 4
-
 [ 590-86-3 ]
[ 590-86-3 ]

-
 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1.1: L-proline / acetonitrile / 3 h / 0 - 10 °C
2.1: LiBr; DBU / acetonitrile / 0.75 h / 5 °C
3.1: H2 / Raney-Ni / methanol / 12 h / 3102.89 Torr
3.2: 70 percent / ethanol / 5 h / 50 °C |
|
- 5
-
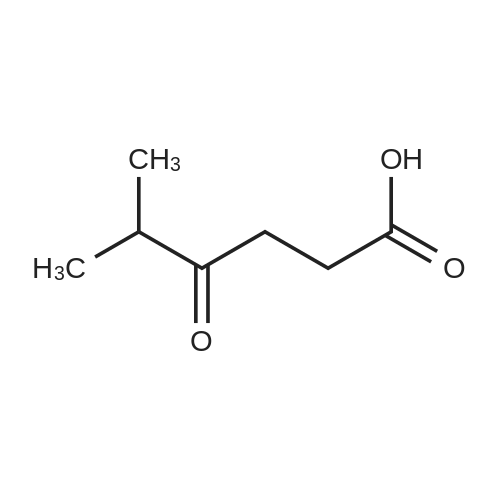 [ 41654-04-0 ]
[ 41654-04-0 ]

-
 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: 84 percent / toluene / 18.5 h / Heating
2: 94 percent / Et3SiH, TiCl4 / CH2Cl2 / a) -78 deg C, 1.5 h, b) r.t., 12 h
3: 81 percent / Na, liq. NH3 / ethanol; tetrahydrofuran / -33 deg C to r.t., 4 h |
|
|
Multi-step reaction with 4 steps
1.1: sulfuric acid / 20 °C
2.1: titanium(IV) tetraethanolate / 72 °C / Inert atmosphere
3.1: [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; 2-Amino-2-methyl-1-propanol; potassium <i>tert</i>-butylate / isopropyl alcohol / 50 °C / Molecular sieve
4.1: hydrogenchloride / methanol / 20 °C
4.2: 0.17 h / pH > 11 |
|
Reference:
[1]Burgess, Laurence E.; Meyers, A. I.
[Journal of Organic Chemistry, 1992, vol. 57, # 6, p. 1656 - 1662]
[2]Guijarro, David; Pablo, Oscar; Yus, Miguel
[Journal of Organic Chemistry, 2013, vol. 78, # 8, p. 3647 - 3654]
- 6
-
 [ 137869-71-7 ]
[ 137869-71-7 ]

-
 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: 94 percent / Et3SiH, TiCl4 / CH2Cl2 / a) -78 deg C, 1.5 h, b) r.t., 12 h
2: 81 percent / Na, liq. NH3 / ethanol; tetrahydrofuran / -33 deg C to r.t., 4 h |
|
- 7
-
ethyl 4-[(S)-tert-butylsulfinylimino]-5-methylhexanoate
[ No CAS ]

-
 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1.1: [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; 2-Amino-2-methyl-1-propanol; potassium <i>tert</i>-butylate / isopropyl alcohol / 50 °C / Molecular sieve
2.1: hydrogenchloride / methanol / 20 °C
2.2: 0.17 h / pH > 11 |
|
- 8
-
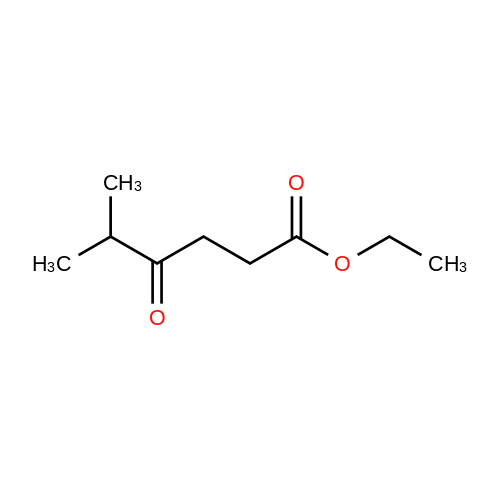 [ 54857-48-6 ]
[ 54857-48-6 ]

-
 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1.1: titanium(IV) tetraethanolate / 72 °C / Inert atmosphere
2.1: [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; 2-Amino-2-methyl-1-propanol; potassium <i>tert</i>-butylate / isopropyl alcohol / 50 °C / Molecular sieve
3.1: hydrogenchloride / methanol / 20 °C
3.2: 0.17 h / pH > 11 |
|
- 9
-
C13H27NO3S
[ No CAS ]

-
 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Stage #1: C13H27NO3S With hydrogenchloride In methanol at 20℃;
Stage #2: With ammonia; ammonium chloride; sodium hydroxide In water for 0.166667h; |
|
- 10
-
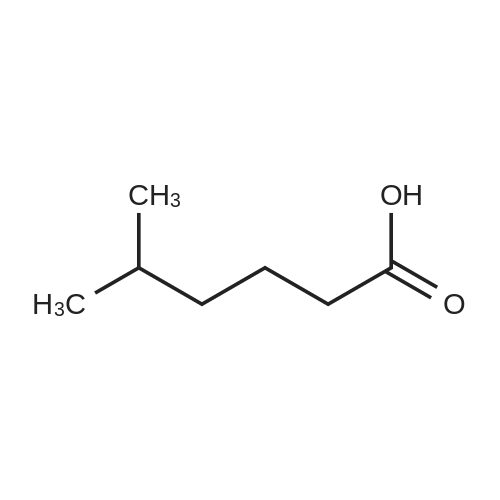 [ 628-46-6 ]
[ 628-46-6 ]

-
 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1.1: 1,1'-carbonyldiimidazole / tetrahydrofuran / 20 °C
1.2: 20 °C
2.1: tetrahydrofuran / 20 °C
3.1: C47H47ClIrN3O5 / 12 h / 20 °C |
|
Reference:
[1]Wang, Hao; Park, Yoonsu; Bai, Ziqian; Chang, Sukbok; He, Gang; Chen, Gong
[Journal of the American Chemical Society, 2019, vol. 141, # 17, p. 7194 - 7201]
- 11
-
C7H15NO2
[ No CAS ]

-
 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: tetrahydrofuran / 20 °C
2: C47H47ClIrN3O5 / 12 h / 20 °C |
|
Reference:
[1]Wang, Hao; Park, Yoonsu; Bai, Ziqian; Chang, Sukbok; He, Gang; Chen, Gong
[Journal of the American Chemical Society, 2019, vol. 141, # 17, p. 7194 - 7201]
- 12
-
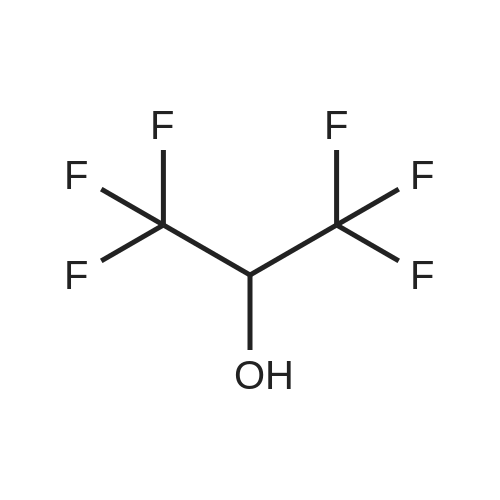 [ 920-66-1 ]
[ 920-66-1 ]

-
C8H13NO3
[ No CAS ]

-
C10H15F6NO2
[ No CAS ]

-
 [ 139564-41-3 ]
[ 139564-41-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 1: 45%
2: 52% |
With C47H47ClIrN3O5 at 20℃; for 12h; enantioselective reaction; |
|
Reference:
[1]Wang, Hao; Park, Yoonsu; Bai, Ziqian; Chang, Sukbok; He, Gang; Chen, Gong
[Journal of the American Chemical Society, 2019, vol. 141, # 17, p. 7194 - 7201]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping









