| 83% |
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In ethyl acetate; acetonitrile; at 0 - 25℃; for 3.0h; |
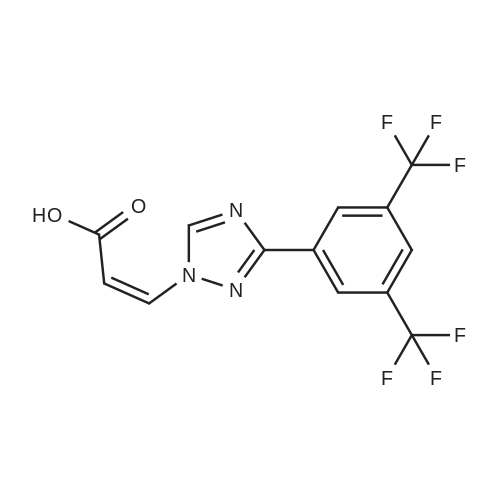
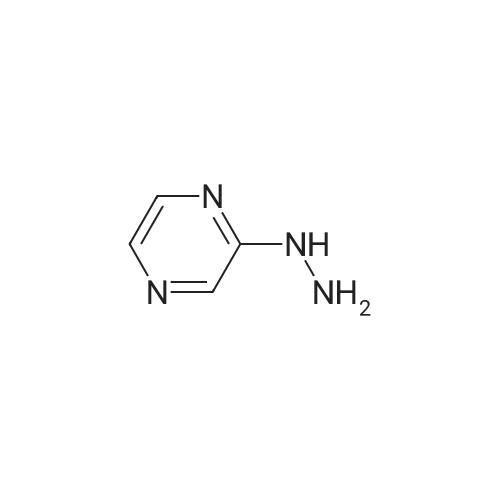
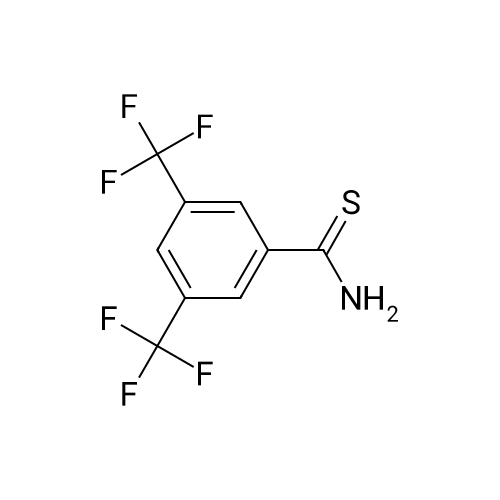
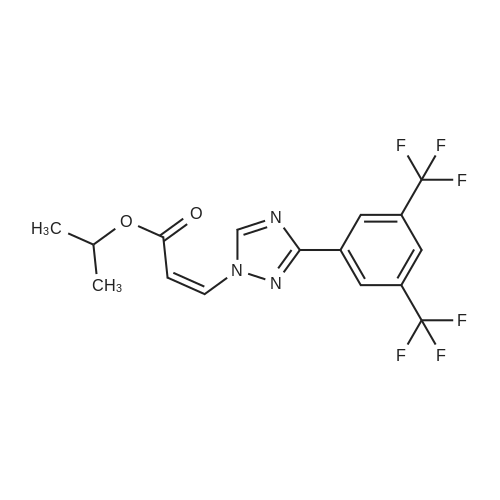
In a 3-L, 3-necked, round-bottomed flask were charged 60.0 gr (Z)-3-(3-(3,5- bis(trifluoromethyl)phenyl)- 1 H- 1,2, 4-triazol- 1 -yl)acrylic acid (SLN- 105, prepared according to examples 27), Ethyl acetate (0.42 lit, 7V) and Acetonitrile (0.3 lit,5V) at 20-25°C. Charged 2-hydrazino pyrazine (19.8 gr, 1.05 eq) then cooled to 0 to 5°C. Charged EDC .HC1 (1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride) (49. lgr 1 .Seq) at 0 to 5°C. The reaction mass was stirred for 3hrs and monitored by HPLC (till SLN-105 NMT 1.0percent). Once the reaction completes, charge water (0.2lit, 2V) and stirred for 15-30 mm at 15-20°C, settled and separated the organic layer. Collected the organic layer and washed with sodium bicarbonate solution (0.Slit, SV). Finally washed the organic layer with water (0.2lit, 2 V) and combined the collected organic layer containing the product. The solvent is distilled off under vacuum at 50 to 60°C for 30 mm. To the obtained solid, added absolute Ethanol (0.6lit, 1OV) and stirred for 30mm at 20-25°C then cooled to 0-5°C and stirred for 1 hr at 0-5°C. Filtered the compound under vacuum at 20-25°C and washed with Ethanol (0.2lit, 2V). The wet cake was dried at 55-60°C under vacuum (600 to 700 mm Hg) for 4 hrs. (Yield 83percent). |
| 16% |
With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine; In dichloromethane; ethyl acetate; at -40℃; for 0.5h; |
Example 2: Synthesis of (Z)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-lH-l,2,4-triazol- 1 -yl)-N'-(pyrazin-2-yl)acr lohydrazide (1-3 .A 50-mL, 3-necked, round-bottomed flask was charged with a suspension of (Z)-3-(3- (3,5-bis(trifluoromethyl)phenyl)-lH-l,2,4-triazol-l-yl)acrylic acid (0.200 g) in 1 :1 CH2C12: AcOEt (25 mL). 2-Hydrazinopyrazine (0.062 g) was added at -40 °C followed by T3P (50percent) (0.432g) and DIPEA (0.147 g). The reaction mixture was stirred for 30 min at -40 °C before being concentrated under reduced pressure (35 °C, 20 mmHg). The crude oil was purified by preparative TLC using 5percent MeOH in CH2C12 as mobile phase (under ammonia atmosphere) to afford 40 mg (yield: 16percent) of (Z)-3-(3-(355-bis(trifluoromethyl)phenyl)-lH-l,2,4-triazol-l- yl)-N'-(pyrazin-2-yl)acrylohydrazide. 1H NMR (400 MHz, DMSO-d6) delta ,10.53 (s, 1H), 9.59 (s, 1H), 9.14 (s, 1H), 8.53 (s, 2H), 8.29 (s, 1H), 8.13 (s, 1H), 8.06-8.07 (m, 1H), 7.92-7.93 (d, J=2.8 Hz, 1H), 7.51-7.53 (d, J=10.4 Hz, 1H), 6.07-6.10 (d, J=10.4 Etazeta,IotaEta); LCMS for CnHi2F6N70 [M+H]+ predicted: 444.31, found: 444.49 (RT 2.70 min, purity: 95.78percent). |
| 7 g |
With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine; In ethyl acetate; acetonitrile; at 0℃; for 2.5h; |
Example-4: Preparation of Selinexor (0507) (0508) (Z)-3-(3-(3,5-bis(trifluoromethyl)phenyl)-1 H-1 ,2,4-triazol-1 -yl)acrylic acid (10 g) was combined with a mixture of acetonitrile (1 00 mL) and ethyl acetate (50 mL) then added the 2-hydrazinylpyrazine (3.76 g) and stirred for 5 min. Reaction mixture was cooled to 0°C and diisopropyl ethyl amine (16.63 ml) and then Propylphosphonic anhydride (T3P, 33.31 mL) was added at 0°C and stirred the reaction mixture for 2.5 hours at the same temperature. After completion of the reaction, the reaction mixture was quenched with cold water (100 mL) and extracted the product with ethyl acetate (2 x 150 mL). The combined organic layer was dried over sodium sulphate and evaporated the solvent under vacuum at 40°C to obtain the crude product as yellow syrup. The obtained crude product was combined with dichloromethane (1 00 mL) and filtered the solid and washed with dichloromethane (2 x 50 mL). The solid was dried under vacuum at 40°C to obtain the title compound with purity by HPLC of 99.86percent. Yield : 7 g |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping