| 41% |
With caesium carbonate In N,N-dimethyl-formamide; acetonitrile at 20℃; for 2.5h; |
10.4
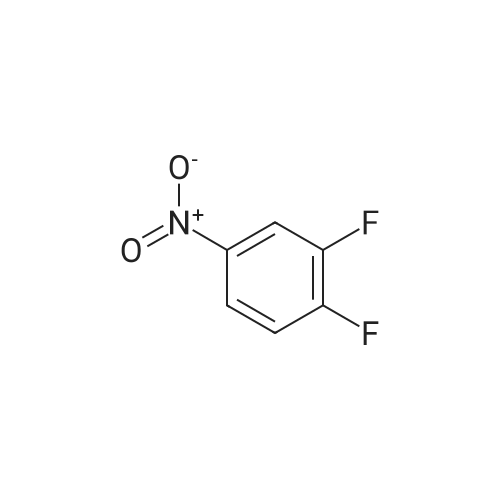
To a round bottom flask equipped with a magnetic stir bar was added 7,8,10,11,13,14- Hexahydro- lH-6,9, 12, 15-tetraoxa- 1 -aza-cyclododeca[b]naphthalen-4-one (12.2g, 43.3mmol, l .Oeq. ), acetonitrile (150ml), DMF (150ml) and cesium carbonate (28.2g, 86.5mmol, 2.0eq). The mixture was stirred at room temperature for 30 minutes at which time 1, 2-difluoro- 4-nitro-benzene (7.57g, 47.6mmoL, 1.1 eq) was added over a 10 minute period. After 2 hours the reaction was complete at which time 75% of the MeCN and DMF was removed and the resulting solution was poured over into ice water. The solid was filtered and dried and further columned with a biotage system. The eluent was 1 :3 ethyl acetate/hexane. Removal of the solvent afforded 4-(2-Fluoro-4-nitro-phenoxy)-7,8, 10,11,13,14-hexahydro-6,9, 12, 15-tetraoxa- l-aza-cyclodo-deca[b]naphthalene as a pale green solid (7.4g, 41% yield). ^-NMR (400MHz, DMSO): 8.58 (s, 1H), 7.80 (s, 1H), 7.50 (s, 1H), 7.04 (t, 1H), 6.88 (m, 1H), 6.50 (m, 2H), 4.35 (s, 4H), 3.87 (d, 4H), 3.62 (s, 4H). LC/MS Calcd for (M+H)+ 431.4, found 431.5. |
| 41% |
With caesium carbonate In N,N-dimethyl-formamide; acetonitrile at 20℃; for 2.66667h; |
10.4 Step 4 4-(2-Fluoro-4-nitro-phenoxy)-7,8,10,11,13,14-hexahydro-6,9,12,15-tetraoxa-1-aza-cyclododeca[b]naphthalene
Step 4 4-(2-Fluoro-4-nitro-phenoxy)-7,8,10,11,13,14-hexahydro-6,9,12,15-tetraoxa-1-aza-cyclododeca[b]naphthalene To a round bottom flask equipped with a magnetic stir bar was added 7,8,10,11,13,14-Hexahydro-1H-6,9,12,15-tetraoxa-1-aza-cyclododeca[b]naphthalen-4-one (12.2 g, 43.3 mmol, 1.0 eq.), acetonitrile (150 ml), DMF (150 ml) and cesium carbonate (28.2 g, 86.5 mmol, 2.0 eq). The mixture was stirred at room temperature for 30 minutes at which time 1,2-difluoro-4-nitro-benzene (7.57 g, 47.6 mmoL, 1.1 eq) was added over a 10 minute period. After 2 hours the reaction was complete at which time 75% of the MeCN and DMF was removed and the resulting solution was poured over into ice water. The solid was filtered and dried and further columned with a biotage system. The eluent was 1:3 ethyl acetate/hexane. Removal of the solvent afforded 4-(2-Fluoro-4-nitro-phenoxy)-7,8,10,11,13,14-hexahydro-6,9,12,15-tetraoxa-1-aza-cyclodo-deca[b]naphthalene as a pale green solid (7.4 g, 41% yield). 1H-NMR (400 MHz, DMSO): 8.58 (s, 1H), 7.80 (s, 1H), 7.50 (s, 1H), 7.04 (t, 1H), 6.88 (m, 1H), 6.50 (m, 2H), 4.35 (s, 4H), 3.87 (d, 4H), 3.62 (s, 4H). LC/MS Calcd for (M+H)+ 431.4, found 431.5. |
| 41% |
Stage #1: 7,8,10,11,13,14-hexahydro-1H-6,9,12,15-tetraoxa-1-aza-cyclododeca[b]naphthalen-4-one With caesium carbonate In N,N-dimethyl-formamide; acetonitrile at 20℃; for 0.5h;
Stage #2: 3,4-difluoronitrobenzene In N,N-dimethyl-formamide; acetonitrile for 2h; |
10.4 Step 4: 4- (2-Fluoro-4-nitro-phenoxy) -7,8,10,11,13,14-hexahydro-6,9,12,15-tetraoxy- -cyclododecane [b] naphthalene
A round bottom flask equipped with a magnetic stirrer was charged7,8,10,11,13,14-hexahydro-1H-6,9,12,15-tetraoxo-1-aza-cyclododecane [b](12.2 g, 43.3 mmol, 1.0 eq),Acetonitrile (150 ml),DMF (150 ml) and cesium carbonate (28.2 g, 86.5 mmol, 2.0 eq).The resulting mixture was stirred at room temperature for 30 minutes,then,With 10 minutes,1,2-Fluoro-4-nitro-benzene (7.57 g, 47.6 mmol, 1.1 eq) was added.After 2 hours, the reaction was 75% complete,The acetonitrile and DMF were removed and then poured into ice water.The solid was filtered, dried, and passed through a column (available from Biotage). The eluent was 1: 3 ethyl acetate / n-hexane. Removal of the solvent gave 4- (2-fluoro-4-nitro-phenoxy) -7,8,10,11,13,14-hexahydro-6,9,12,15-tetraoxy- 1-aza-cyclododecane [b] naphthalene (7.4 g, yield 41%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping