| 88.8% |
With potassium hydroxide In water for 2h; Reflux; |
1.1-2
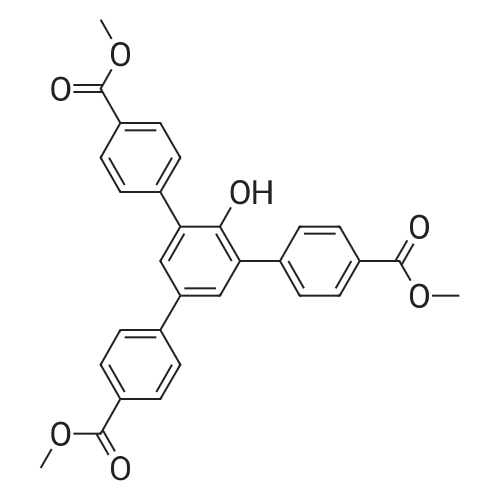
2,4,6-tris (4-methoxycarbonyl) phenol (187.00 g), potassium hydroxide (76.08 g) manufactured by Kanto Chemical Co., Ltd., pure water (1870 mL) were placed in a reaction vessel and heated under reflux Followed by stirring. After 2 hours, the reaction was traced by TLC, and the disappearance of the raw material was confirmed. After allowing to cool to room temperature, insoluble matter was removed by filtration. Concentrated hydrochloric acid was added dropwise to the resulting mother liquor under ice cooling, and the pH was adjusted to 2 (Univ.). The crystallized crystals were centrifuged (rotation speed: 3100 rpm, time: 5 minutes, temperature: 4 ° C.), the mother liquor was removed, and the crystals were washed 5 times with pure water. The obtained wet crystals were air-dried at 60 ° C. for 18 hours and then pulverized in a mortar. This powdered crystal was further dispersed and washed with isopropyl ether and dried to obtain 151.83 g of the desired product as a slight brown powder. (Yield: 88.8%) |
| 151.83 g |
With water; potassium hydroxide for 2h; Reflux; |
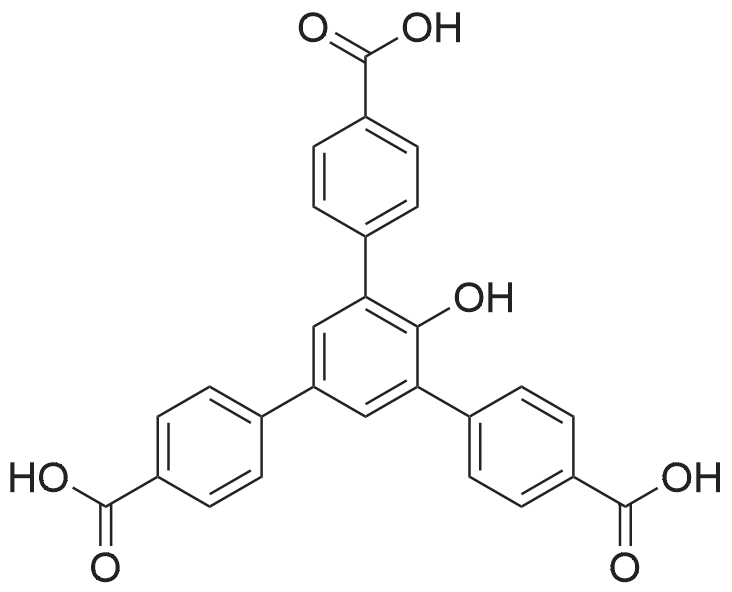
1.1-2 [Step 1-2: Synthesis of 2,4,6-tris-(4-carboxyphenyl)phenol]
[Step 1-2: Synthesis of 2,4,6-tris-(4-carboxyphenyl)phenol] In a reactor there were placed 2,4,6-tris(4-methoxycarbonyl)phenol (187.00 g), potassium hydroxide by Kanto Kagaku Co., Ltd. (76.08 g) and purified water (1870 mL), and the mixture was stirred for 2 hours while heating to reflux to obtain a reaction mixture. The reaction mixture was then subjected to TLC to monitor the reaction. It was thus confirmed that the 2,4,6-tris(4-methoxycarbonyl)phenol starting material had disappeared. After allowing the reaction mixture to cool to room temperature, the insoluble portion was filtered out. Concentrated hydrochloric acid was added dropwise to the obtained mother liquor while cooling on ice, to adjust the pH to 2 (Univ). The precipitated crystals were centrifuged (rotational speed: 3100 rpm, time: 5 minutes, temperature: 4°C) and the mother liquor was removed. Next, the obtained wet crystals were rinsed 5 times with purified water and dried with forced air at 60°C for 18 hours. The obtained crystals were pulverized with a mortar to obtain powder crystals. The powder crystals were dispersed and rinsed with isopropyl ether and dried, to obtain 151.83 g of a faint brown powder (2,4,6-tris-(4-carboxyphenyl)phenol) as the target compound. (Yield: 88.8 wt%) The 1H-NMR, 13C-NMR and MS results for the obtained 2,4,6-tris-(4-carboxyphenyl)phenol were as follows. 1H-NMR (300 MHz, DMSO-d6):δ = 12.93 (bs, 2H,COOH), 8.95 (bs, 1H,COOH), 8.02 (d, J = 8.2 Hz, 4H,Ar), 7.98 (d, J = 8.4 Hz, 2H,Ar), 7.88 (d, J = 8.4 Hz, 2H,Ar), 7.77 (d, J = 8.2 Hz, 4H,Ar), 7.65 (s, 2H,Ar) 13C-NMR (300 MHz, DMSO-d6):δ = 167.26(COOH), 167.19(COOH), 151.15(Ar), 143.69(Ar), 142.81(Ar), 131.60(Ar), 130.83(Ar), 129.90(Ar), 129.72(Ar), 129.43(Ar), 129.26(Ar), 129.09(Ar), 128.91(Ar), 126.74(Ar) MS: m/z = 454.18(M+) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping