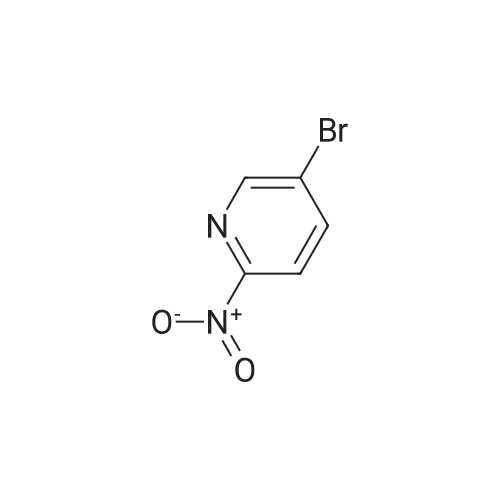
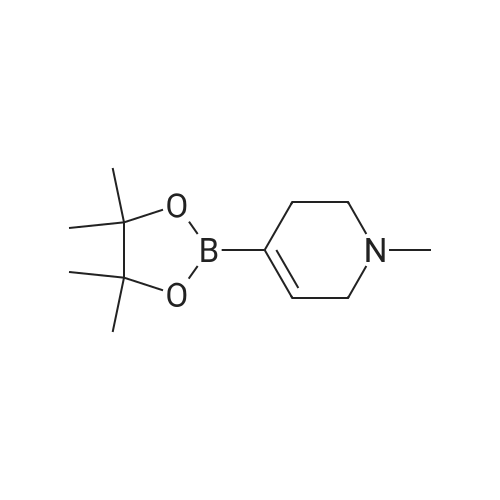
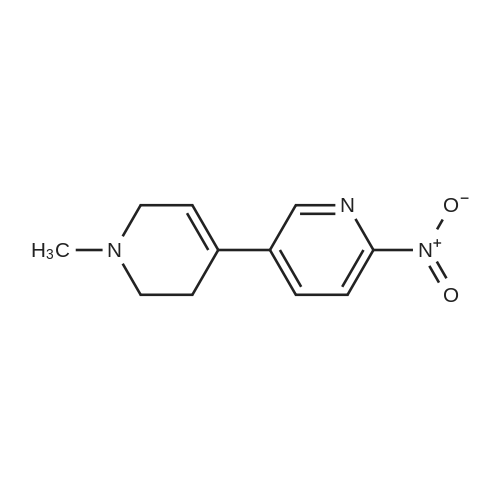
| 26% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; caesium carbonate; In 1,4-dioxane; water; at 85℃; for 12h; |
Add to the reaction flask5-bromo-2-nitropyridine (20.3 g, 0.1 mol)1-methyl-4- (4,4,5,5-tetramethyl-1,3,2-dioxaboride-2-yl) -1,2,3,6-tetrahydropyridine (22.3 g , 0.1 mol), cesium carbonate (65 g, 0.2 mol), Pd (dppf) Cl2 (7.33 g,0.01 mol) and dioxane / water (250 mL / 30 mL).The mixture was heated and stirred at 85 ° C for 12 hours, cooled to room temperature, concentrated under reduced pressure,The resulting residue was purified by column chromatography (petroleum ether / ethyl acetate = 1: 1)To give the title compound (5.7 g, white solid) in 26percent yield. |
| 26% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; caesium carbonate; In 1,4-dioxane; water; at 85℃; for 12h;Inert atmosphere; |
Cesium carbonate ([Cs2CO3] 66.00g, 0.2mol) and Pd(dppf)Cl2 (7.33g, 0.01mol) were added to a solution of 82 5-bromo-2-nitropyridine (20.30g, 0.1mol) and 110 <strong>[454482-11-2]1-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,2,3,6-tetrahydropyridine</strong> (22.30g, 0.1mol) in 111 dioxane/112 H2O (250 mL/30mL). The mixture was allowed to stir at 85°C for 12h under nitrogen (N2). Then the solution was cooled to RT, concentrated under a vacuum, and purified by silica gel column chromatography (from 102 PE/86 EA=1:1 to 103 DCM/87 MeOH=20:1) to obtain 113 1?-methyl-6-nitro-1?,2?,3?,6?-tetrahydro-3,4?-bipyridine (5.70g; yield, 26percent) as a white solid. Next, Pd/C (0.10g) was added to the solution of 1?-methyl-6-nitro-1?,2?,3?,6?-tetrahydro-3,4?-bipyridine (657.0mg, 3.0mmol) in EA/MeOH (10 mL/10mL). The mixture was degassed by flushing with H2, stirred at RT under a H2 atmosphere for 2h, and then filtered and concentrated under a vacuum to obtain INT-4 (550.0mg; yield, 96percent) as a white solid. ESI-MS: m/z 192.2 [M+H]+. |
|
With sodium carbonate;tetrakis(triphenylphosphine) palladium(0); In 1,4-dioxane; water; at 100℃; for 10h; |
To a round-bottomed flask equipped with a stirring bar, l-methyl-4-(4,4,5,5- tetramethyl-l,3,2-dioxaborolan-2-yl)-l,2,3,6-tetrahydropyridine (1.50 g, 6.72 mmol), 5- bromo-2-nitropyridine (1.64 g, 8.07 mmol), Pd(PPh3)4 (388 mg, 0.336 mmol), Na2C03 aqueous solution (1.0 N, 20.2 mL, 20.2 mmol), dioxane (60.6 mL) were added. The reaction mixture was heated at 100 °C for 10 hrs. CH2CI2 (200 mL) was added to the resulting mixture was washed with water (30 mL X 3). CH2CI2 (200 mL) was added and the resulting mixture was washed with water (30 mL X 3), brine (30 mL X 1), dried over MgS04, filtered, and removed solvent in vacuo. Silica gel column chromatography (MeOH: DCM = 5: 95) gave 5- (l-methyl-l,2,3,6-tetrahydropyridin-4-yl)-2-nitropyridine (130a) as a yellow solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping