| 63% |
With hydrogenchloride In ethanol |
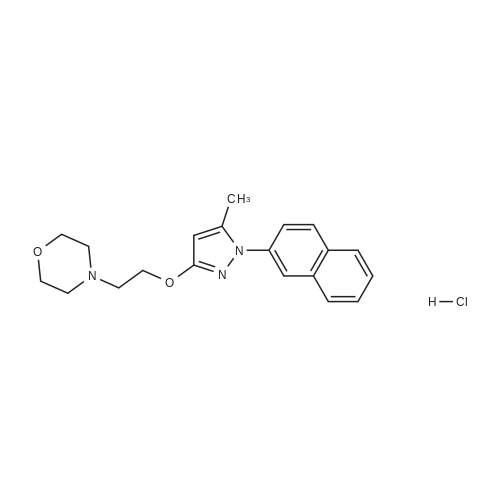
1 Example 1 : Synthesis of 4-{2-[5-Methyl-1-(naphthalen-2-yl)-1H-pyrazol-3- yloxy]ethyl} morpholine hydrochloride (compound 63-HCI)
Compound 63 Compound 63-HCI Compound 63 can be prepared as disclosed in the previous application WO2006/021462. Its hydrochloride can be obtained according the following procedure:Compound 63 (6.39 g) was dissolved in ethanol saturated with HCI. The mixture was stirred then for some minutes and evaporated to dryness. The residue was crystallized from isopropanol. The mother liquors from the first crystallization afforded a second crystallization by concentrating. Both crystallizations taken together yielded 5.24 g (63 %) of the corresponding hydrochloride salt (m.p. = 197-199°C.)1H-NMR (DMSO-de) δ ppm: 10,85 (bs, 1 H), 7,95 (m, 4H), 7,7 (dd, J=2,2, 8,8 Hz, 1 H), 7,55 (m, 2H), 5,9 (s, 1 H), 4,55 (m, 2H), 3,95 (m, 2H), 3,75 (m, 2H), 3,55-3,4 (m, 4H), 3,2 (m, 2H), 2,35 (s, 3H).HPLC purity: 99.8% |
| 63% |
With hydrogenchloride In ethanol |
1 Example 1. Synthesis of 4-{2-[5-Methyl-1 -(naphthalen-2-yl)-1 H-pyrazol-3- yloxy]ethyl} morpholine (compound 63) and its hydrochloride salt
Compound 63 can be prepared as disclosed in the previous application WO2006/021462. Its hydrochloride can be obtained according the following procedure: Compound 63 (6.39 g) was dissolved in ethanol saturated with HCI, the mixture was stirred then for some minutes and evaporated to dryness. The residue was crystallized from isopropanol. The mother liquors from the first crystallization afforded a second crystallization by concentrating. Both crystallizations taken together yielded 5.24 g (63 %) of the corresponding hydrochloride salt (m.p. = 197-199°C.) 1H-NMR (DMSO-de) δ ppm: 10,85 (bs, 1 H), 7,95 (m, 4H), 7,7 (dd, J=2,2, 8,8 Hz, 1 H), 7,55 (m, 2H), 5,9 (s, 1 H), 4,55 (m, 2H), 3,95 (m, 2H), 3,75 (m, 2H), 3,55-3,4 (m, 4H), 3,2 (m, 2H), 2,35 (s, 3H). HPLC purity: 99.8% |
| 63% |
With hydrogenchloride In ethanol |
1 Example 1. Synthesis of 4-{2-[5-Methyl-1-(naphthalen-2-yl)-1H-pyrazol-3-yloxy]ethyl} morpholine (compound 63) and its hydrochloride salt
Example 1. Synthesis of 4-{2-[5-Methyl-1-(naphthalen-2-yl)-1H-pyrazol-3-yloxy]ethyl} morpholine (compound 63) and its hydrochloride salt (0108) (0109) Compound 63 can be can be prepared as disclosed in the previous application . Its hydrochloride can be obtained according the following procedure: Compound 63 (6,39 g) was dissolved in ethanol saturated with HCl, the mixture was stirred then for some minutes and evaporated to dryness. The residue was crystallized from isopropanol. The mother liquors from the first crystallization afforded a second crystallization by concentrating. Both crystallizations taken together yielded 5,24 g (63 %) of the corresponding hydrochloride salt (m.p. = 197-199°C.) 1H-NMR (DMSO-d6) δ ppm: 10,85 (bs, 1H), 7,95 (m, 4H), 7,7 (dd, J=2,2, 8,8 Hz, 1H), 7,55 (m, 2H), 5,9 (s, 1 H), 4,55 (m, 2H), 3,95 (m, 2H), 3,75 (m, 2H), 3,55-3,4 (m, 4H), 3,2 (m, 2H), 2,35 (s, 3H). HPLC purity: 99.8% |
| 63% |
With hydrogenchloride In ethanol |
1 Example 1
Compound 63 can be can be prepared as disclosed in the previous application . Its hydrochloride can be obtained according the following procedure: Compound 63 (6,39 g) was dissolved in ethanol saturated with HCl, the mixture was stirred then for some minutes and evaporated to dryness. The residue was crystallized from isopropanol. The mother liquors from the first crystallization afforded a second crystallization by concentrating. Both crystallizations taken together yielded 5.24 g (63 %) of the corresponding hydrochloride salt (m.p. = 197-199 °C). 1H-NMR (DMSO-d6) δ ppm: 10,85 (bs, 1H), 7,95 (m, 4H), 7,7 (dd, J=2,2, 8,8 Hz, 1H), 7,55 (m, 2H), 5,9 (s, 1H), 4,55 (m, 2H), 3,95 (m, 2H), 3,75 (m, 2H), 3,55-3,4 (m, 4H), 3,2 (m, 2H), 2,35 (s, 3H). HPLC purity: 99.8% |
| 63% |
With hydrogenchloride In ethanol; water |
1
Compound 63 (6,39 g) was dissolved in ethanol saturated with HC1, the mixture was stirred then for some minutes and evaporated to dryness. The residue was crystallized from isopropanol. The mother liquors from the first crystallization afforded a secondcrystallization by concentrating. Both crystallizations taken together yielded 5.24 g (63 %) of the corresponding hydrochloride salt (m.p. = 197-199 °C). |
| 63% |
With hydrogenchloride In ethanol |
1 Example 1 Synthesis of 4-{2-[5-Methyl-1-(naphthalen-2-yl)-1H-pyrazol-3-yloxy]ethyl}morpholine (compound 63) and its hydrochloride salt
Compound 63 (6.39 g) was dissolved in ethanol saturated with HCl, the mixture was stirred then for some minutes and evaporated to dryness. The residue was crystallized from isopropanol. The mother liquors from the first crystallization afforded a second crystallization by concentrating. Both crystallizations taken together yielded 5.24 g (63%) of the corresponding hydrochloride salt (m.p.=197-199° C.). 1H-NMR (DMSO-d6) δ ppm: 10.85 (bs, 1H), 7.95 (m, 4H), 7.7 (dd, J=2.2, 8.8 Hz, 1H), 7.55 (m, 2H), 5.9 (s, 1H), 4.55 (m, 2H), 3.95 (m, 2H), 3.75 (m, 2H), 3.55-3.4 (m, 4H), 3.2 (m, 2H), 2.35 (s, 3H). |
| 63% |
With hydrogenchloride In ethanol |
1 Example 1 Synthesis of 4-{2-[5-methyl-1 -(naphthalen-2-yl)- 1 H-pyrazol-3-yloxy]ethyl}morpholine (Compound61) and its Hydrochloride Salt
Compound 61 can be can be prepared as disclosed in the previous application W02006/021462. Its hydrochloride can be obtained according the following procedure:Compound 61(6.39 g) was dissolved in ethanol saturated with HC1, the mixture was stirred then for some minutes and evaporated to dryness. The residue was crystallized from isopropanol. The mother liquors from the first crystallization afforded a second crystallization by concentrating. Both crystallizations taken together yielded 5.24 g (63%) of the corresponding hydrochloride salt (m.p.=197-199° C.).1H-NMR (DMSO-d5)δ ppm: 10.85 (bs, 1H), 7.95 (m,4H), 7.7 (dd, J=2.2, 8.8 Hz, 1H), 7.55 (m, 2H), 5.9 (s, 1H),4.55 (m, 2H), 3.95 (m, 2H), 3.75 (m, 2H), 3.55-3.4 (m, 4H),3.2 (m, 2H), 2.35 (s, 3H). HPLC purity: 99.8% |
| 63% |
With hydrogenchloride In ethanol; water |
Example 1 Synthesis of 4-{2-[5-Methyl-1 -(naphthalen-2-yl)-1 H-pyrazol-3-yloxy]ethyl} morpholine (compound 61) and its hydrochloride salt
Compound 61 can be prepared as disclosed in the previous application W02006/021462. Its hydrochloride can be obtained according the followingprocedure:Compound 63 (6.39 g) was dissolved in ethanol saturated with HCl, the mixture was stirred then for some minutes and evaporated to dryness. The residue was crystallized from isopropanol. The mother liquors from the first crystallization afforded a second crystallization by concentrating. Both crystallizations taken together yielded 5.24 g (63%) of the corresponding hydrochloride salt (m.p. = 197-1 99 °C).1H-NMR (DMSO-d6) 6 ppm: 10,85 (bs, 1H), 7,95 (m, 4H), 7,7 (dd, J=2,2, 8,8 Hz,1H),7,55 (m, 2H), 5,9 (s, 1H), 4,55 (m, 2H), 3,95 (m, 2H), 3,75 (m, 2H), 3,55-3,4 (m, 4H),3,2 (m, 2H), 2,35 (s, 3H).HPLC purity: 99.8%. |
| 63% |
With hydrogenchloride In ethanol |
1 Synthesis of 4-{2-[5-Methyl-1 -(naphthalen-2-yI)-1 H-pyrazol-3-yloxy]ethyl}morpholine (compound 63) and its hydrochloride salt
Compound 63 can be prepared as disclosed in the previous application W02006/021462 (Compound 63 is example 61 in W02006/021462). Itshydrochloride can be obtained according the following procedure:Compound 63 (6.39 g) was dissolved in ethanol saturated with HCI, the mixture was stirred then for some minutes and evaporated to dryness. The residue was crystallized from isopropanol. The mother liquors from the first crystallization afforded a second crystallization by concentrating. Both crystallizations taken together yielded 5.24 g (63%) of the corresponding hydrochloride salt (m.p. = 197-1 99 °C).1H-NMR (DMSO-d6) 6 ppm: 10,85 (bs, 1H), 7,95 (m, 4H), 7,7 (dd, J=2,2, 8,8 Hz,1H),7,55 (m, 2H), 5,9 (s, 1 H), 4,55 (m, 2H), 3,95 (m, 2H), 3,75 (m, 2H), 3,55-3,4 (m, 4H),3,2 (m, 2H), 2,35 (s, 3H).HPLC purity: 99.8%. |
| 63% |
With hydrogenchloride In ethanol |
1 Synthesis of 4-{2-[5-Methyl-1 -(naphthalen-2-yl)-1 H-pyrazol-3-yloxy]ethyl }morpholine (compound 61) and its hydrochloride salt
Compound 61 (6,39 g) was dissolved in ethanol saturated with HCI, the mixture was stirred then for some minutes and evaporated to dryness. The residue was crystallized from isopropanol. The mother liquors from the first crystallization afforded a second crystallization by concentrating. Both crystallizations taken together yielded 5.24 g (63%) of the corresponding hydrochloride salt (m.p. = 197-199 C).1H-NMR (DMSO-d6) 6 ppm: 10,85 (bs, 1H), 7,95 (m, 4H), 7,7 (dd, J=2,2, 8,8 Hz,1H),7,55 (m, 2H), 5,9 (s, 1H), 4,55 (m, 2H), 3,95 (m, 2H), 3,75 (m, 2H), 3,55-3,4 (m, 4H),3,2 (m, 2H), 2,35 (s, 3H).HPLC purity: 99.8%. |
| 5.24 g (63%) |
In ethanol |
1 Example 1.
Compound 61 (6.39 g) was dissolved in ethanol saturated with HCl, the mixture was stirred then for some minutes and evaporated to dryness. The residue was crystallized from isopropanol. The mother liquors from the first crystallization afforded a second crystallization by concentrating. Both crystallizations taken together yielded 5.24 g (63%) of the corresponding hydrochloride salt (m.p. = 197-199 °C). 1H-NMR (DMSO-d6) δ ppm: 10.85 (bs, 1H), 7.95 (m, 4H), 7.7 (dd, J=2.2, 8.8 Hz, 1H), 7.55 (m, 2H), 5.9 (s, 1H), 4.55 (m, 2H), 3.95 (m, 2H), 3.75 (m, 2H), 3.55-3.4 (m, 4H), 3.2 (m, 2H), 2.35 (s, 3H). HPLC purity: 99.8% |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping




