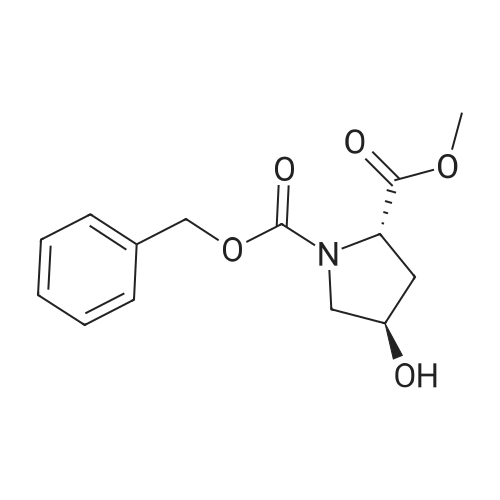
|
With water; sodium hydroxide In methanol at 0℃; for 1h; Inert atmosphere; |
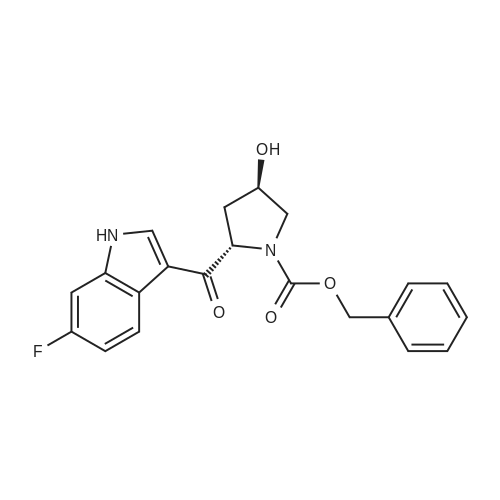
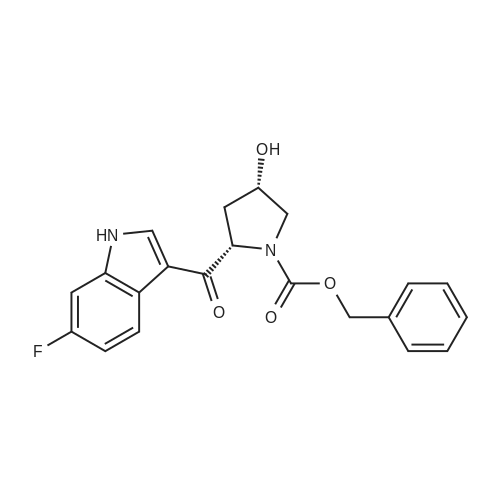
1.XIV
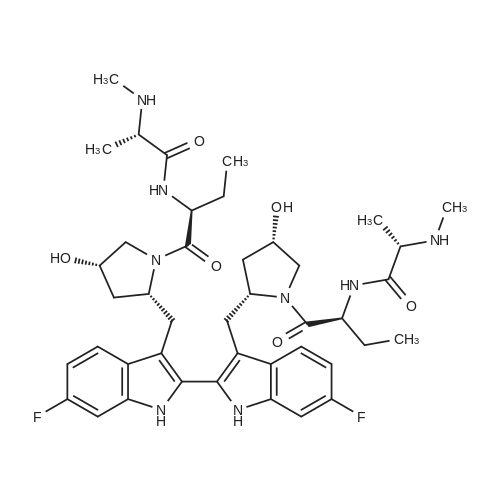
N-{1S-2R-6,6'-Difluoro-3'-4S-hydroxy-1-[2S-(2S-methylamino-propionylamino)-butyryl]-pyrrolidin-2R-ylmethyl}-1H,1'H-[2,2']biindolyl-3-ylmethyl)-4S-hydroxy-pyrrolidine-1-carbonyl]-propyl}-2S-methylamino-propionamide (15): To a solution containing 14 (24 g) in MeOH (200 mL) was added 1 M NaOH (80 mL) at 0° C. The reaction mixture was degassed and maintained under a nitrogen atmosphere wrapped with aluminum foil. The ice-bath was removed. After 60 min, the MeOH was removed in vacuo and the residue was diluted with water (200 mL) and extracted with EtOAc (500 mL). The aqueous phase was separated and back-extracted with EtOAc (2×150 mL). The combined organic extracts were washed with brine and dried over anhydrous Na2SO4, filtered, and concentrated to afford 22.5 g of crude 15 as a light, brown/yellow-colored solid.The crude 15 (22.5 g) was dissolved in MeOH (50 mL) and EtOAc (200 mL). The volume was reduced (50%) by distillation at reduced pressure at 60° C. using a rotary evaporator. MTBE (300 mL) was added and the cloudy solution was warmed to 60° C. After 30 min, the solution was cooled to ambient temperature and then maintained at -5° C.After 16 h, the solid was collected by vacuum filtration and washed with cold 25% EtOAc/MTBE and dried under high vacuum at ambient temperature to afford 16.6 g of 15 as an off-white solid. An additional 5.5 g of 15 was recovered from the filtrate via solvent removal and vacuum drying. 1H NMR (300 MHz, CDCl3): δ11.74 (s, 2H), 8.27 (d, J=8.7 Hz, 2H), 7.71 (dd, J=5.4, 8.4 Hz, 2H), 7.55 (dd, J=2.4, 9.6 Hz, 2H), 6.88 (ddd, J=2.4, 9.3, 9.3 Hz, 2H), 4.62-4.78 (m, 4H), 4.43 (dd, J=9.3, 9.9 Hz, 2H), 4.03 (dd, J=4.8, 11.4 Hz, 2H), 3.80 (d, J=11.4 Hz, 2H), 3.66 (dd, J=2.7, 14.4 Hz, 2H), 3.53 (dd, J=11.4, 14.4 Hz, 2H), 3.11 (q, J=6.9 Hz, 2H), 2.56 (s, 6H), 2.45 (m, 2H), 2.19 (d, J=14.4 Hz, 2H), 1.76-2.10 (m, 6H), 1.59 (br s, 2H), 1.39 (d, J=6.9 Hz, 6H), 1.22-1.38 (m, 2H), 1.07 (t, J=7.2 Hz, 6H) ppm; 13C NMR (75 MHz, d6-DMSO): 6175.2, 172.8, 161.6, 158.5, 137.3, 137.2, 128.4, 128.3, 126.4, 120.8, 120.6, 109.4, 108.7, 108.4, 98.4, 98.0, 70.8, 60.2, 59.9, 56.6, 51.8, 36.4, 35.3, 28.3, 25.6, 20.0, 10.6 ppm. Mass spectrum (ESI), m/z 807.5 [(M)+; calcd for C42H56F2N8O6: 806.9]. |
| 22.1 mg |
With methanol; sodium hydroxide In water at 0℃; for 1h; Inert atmosphere; |
1 N-{(1S)-[(2R)-(6,6'-difluoro-3'-{(4S)-hydroxy-1-[(2S)-[(2S)-methylaminopropionylamino]butyryl]pyrrolidin-(2R)-2-ylmethyl}-1H,1 'H-[2,2']biindolyl-3-ylmethyl)-(4S)-hydroxypyrrolidine-1-carbonyl]propyl}-(2S)-methylaminopropionamide (15)
To a solution containing 14 (24 g) in MeOH (200 mL) was added 1 M NaOH (80 mL) at 0 °C. The reaction mixture was degassed and maintained under a nitrogen atmosphere wrapped with aluminum foil. The ice-bath was removed. After 60 min, the MeOH was removed in vacuo and the residue was diluted with water (200 mL) and extracted with EtOAc (500 mL). The aqueous phase was separated and back-extracted with EtOAc (2 x 150 mL). The combined organic extracts were washed with brine and dried over anhydrous Na2S04, filtered, and concentrated to afford 22.5 g of crude 15 as a light, brown/yellow-colored solid. [0075] The crude 15 (22.5 g) was dissolved in MeOH (50 mL) and EtOAc (200 mL). The volume was reduced (50%) by distillation at reduced pressure at 60 °C using a rotary evaporator. MTBE (300 mL) was added and the cloudy solution was warmed to 60 °C. After 30 min, the solution was cooled to ambient temperature and then maintained at -5 °C. After 16 h, the solid was collected by vacuum filtration and washed with cold 25% EtOAc/MTBE and dried under high vacuum at ambient temperature to afford 16.6 g of 15 as an off-white solid. An additional 5.5 g of 15 was recovered from the filtrate via solvent removal and vacuum drying. 1H NMR (300 MHz, CDCl3): 511.74 (s, 2H), 8.27 (d, J= 8.7 Hz, 2H), 7.71 (dd, J= 5.4, 8.4 Hz, 2H), 7.55 (dd, J =2.4, 9.6 Hz, 2H), 6.88 (ddd, J= 2.4, 9.3, 9.3 Hz, 2H), 4.62-4.78 (m, 4H), 4.43 (dd, J= 9.3, 9.9 Hz, 2H), 4.03 (dd, J= 4.8, 11.4 Hz, 2H), 3.80 (d, J = 11.4 Hz, 2H), 3.66 (dd, J= 2.7, 14.4 Hz, 2H), 3.53 (dd, J = 11.4, 14.4 Hz, 2H), 3.11 (q, J = 6.9 Hz, 2H), 2.56 (s, 6H), 2.45 (m, 2H), 2.19 (d, J= 14.4 Hz, 2H), 1.76-2.10 (m, 6H), 1.59 (br s, 2H), 1.39 (d, J= 6.9 Hz, 6H), 1.22-1.38 (m, 2H), 1.07 (t, J = 7.2 Hz, 6H) ppm; 13C NMR (75 MHz, d6- DMSO): 5175.2, 172.8, 161.6, 158.5, 137.3, 137.2, 128.4, 128.3, 126.4, 120.8, 120.6, 109.4, 108.7, 108.4, 98.4, 98.0, 70.8, 60.2, 59.9, 56.6, 51.8, 36.4, 35.3, 28.3, 25.6, 20.0, 10.6 ppm. Mass spectrum (ESI), m/z 807.5 [(M)+; calcd for C42H56F2N806: 806.9] |
| 0.87 kg |
With sodium hydroxide In methanol; water at 0 - 20℃; for 4h; Inert atmosphere; Large scale; |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping