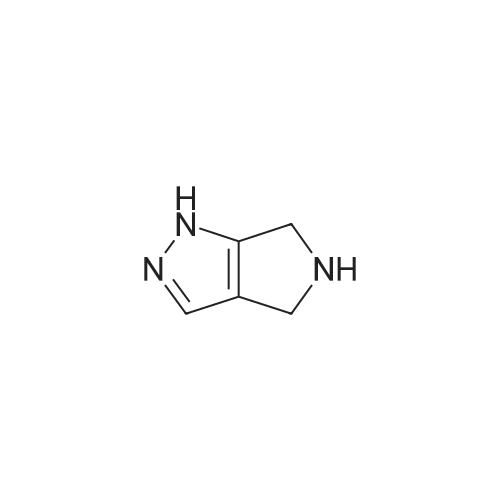
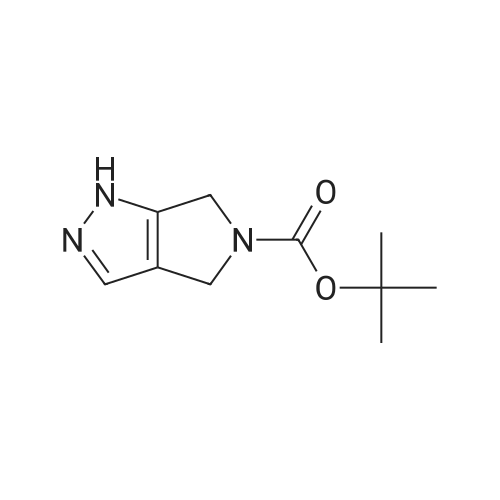
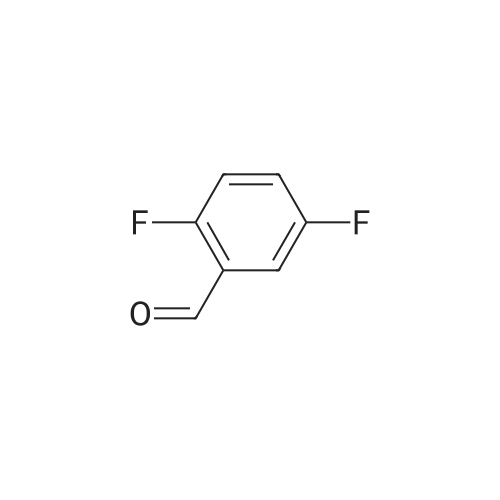
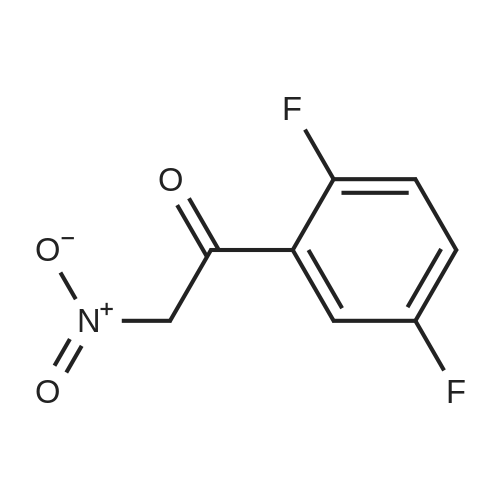
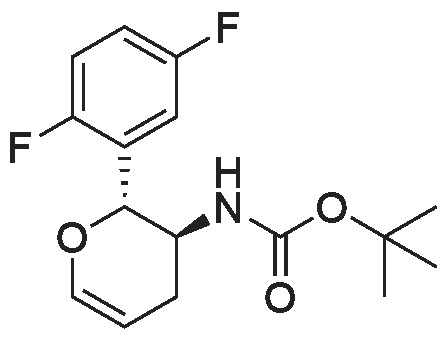
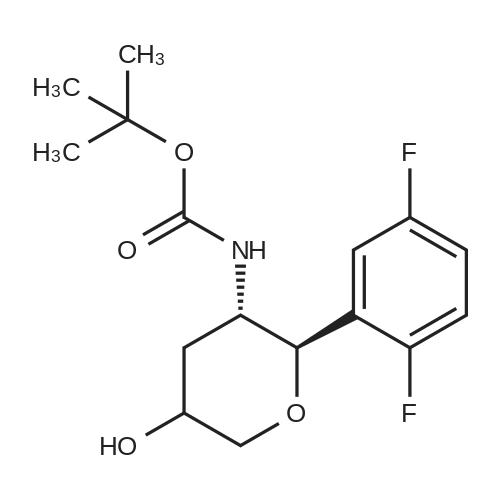
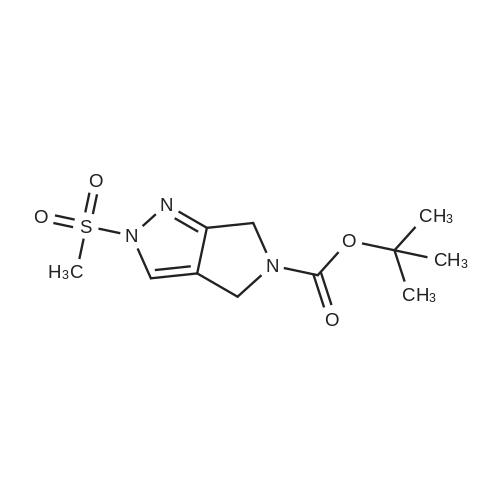
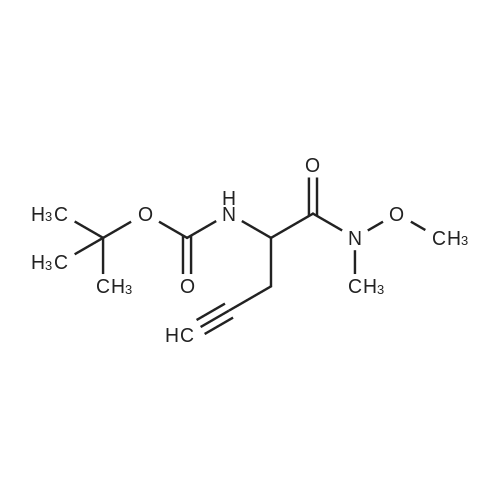
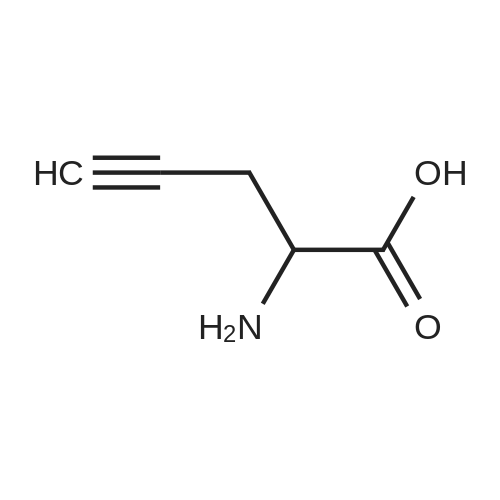
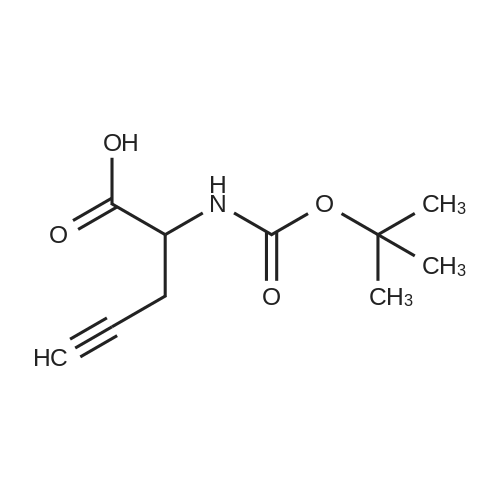
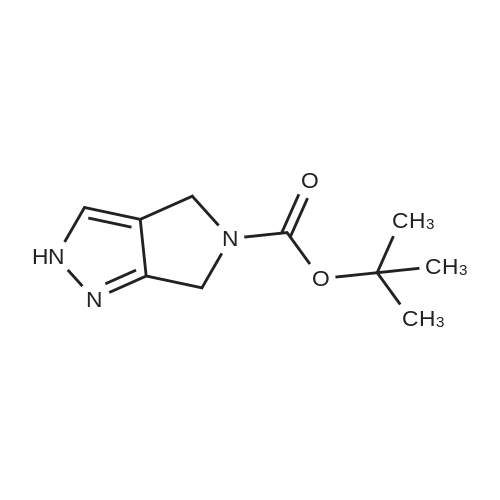
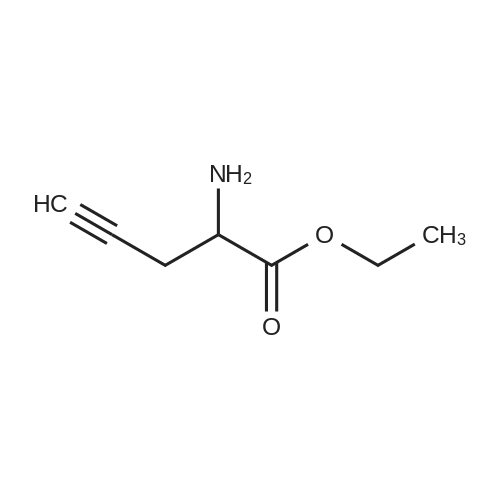
| 70.9% |
With benzenesulfonic acid In acetonitrile at 5 - 25℃; for 17h; Large scale; |
11 Embodiment 11: austria Geleg sandbank IV preparation method
The benzene sulfonic acid 2.85Kg added to 30L of acetonitrile,Circulating frozen saline bath to 5 ± 5 ,Join the Austrian below 15 The Ketagutin intermediate I 3Kg, which can be prepared as in Examples 3, 4, 5, 6, 7, 8 or 9,Stir at this temperature for 1 hour and evacuateFrozen brine,Heated to 25 ± 5 for 16 hours;The solvent was concentrated in vacuo below 35 ° C,The residue was added to 15 L of ethyl acetate and15L 5 ± 5 water to dissolve clear,Layered,Discard the organic layerThe aqueous layer was adjusted to pH = 8 with 7% sodium bicarbonate solution,Add 15L BEthyl acetate,Layered,The aqueous layer was extracted three more times with 12 L of ethyl acetate,The combined organic layers,Dried over anhydrous sodium sulfate,Pressure filtration,The filtrate was vacuum distilled at 35 ° C to about 15 L remaining,The residual suspension is heated to 30-35 ° C,Join 12L methanol solution,Add activated carbon 240g stirring 0.5 to 1 hour,Pressure filtration, the filtrate was heated to 30 ~ 35 ,N-Heptane 12L was added dropwise,Stirred for 0.5 hours,Down to 20 ~ 25 ° C for 3 to 4 hours,Centrifuge,Wet product with 10 L of ethyl acetate:N-heptane = 1: 4 rinse,40 vacuum dried for 12 hours to give a white solid 2.0Kg;The white solid was added to 10 L of ethyl acetate,Heated to 30 under pressure filtration,35 ° CAdd 4.4L methanol dissolved,Pressure filtration,The filtrate was heated to 30 ~ 35 ,Add n-heptane 4L, drop to 20 ~ 25 stirringMix for 3 hours, centrifuged and dried at 40 for 12 hours in vacuum to obtain 1.7 kg of alogliptin IV in a yield of 70.9%. |
|
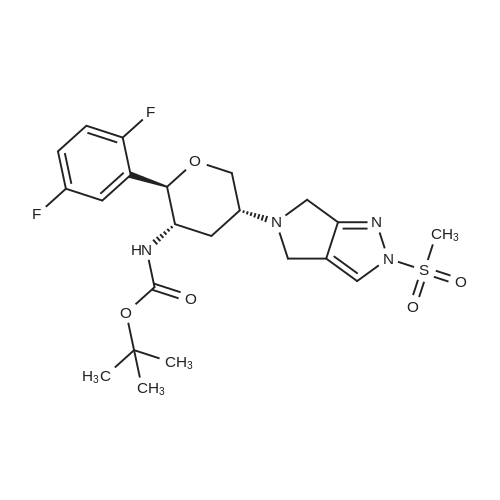
Stage #1: tert-butyl {(2R,3S,5R)-2-(2,5-difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-yl}carbamate With trifluoroacetic acid In dichloromethane at 20℃; for 2h;
Stage #2: With ammonium hydroxide In methanol; dichloromethane |
1.B
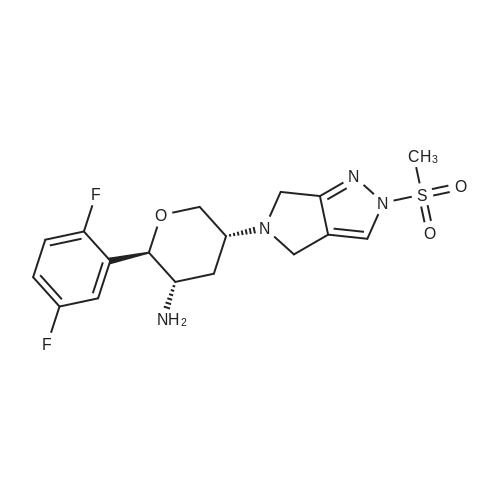
Step B: (2R,3S,5R)-2-(2,5-Difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine; Removal of the BOC group in the product from Step A (13.78 g, 27.67 mmol) was accomplished with trifluoroacetic acid (100 ml) in dichloromethane (200 mL) at room temperature. After stirring for 2 h, the reaction was concentrated and neutralized with 25% MeOH and 2.5% ammonium hydroxide in dichloromethane. Solvents were removed under reduced pressure and the resulting crude material was purified on a 65i Biotage column eluting with 1.25-5% MeOH and 0.125-0.5% ammonium hydroxide in dichloromethane. The isolated material was further purified by recrystallization from 5:1 EtOAc/CH2Cl2 at 60° C. The crystalline product was washed with cold 2:1EtOAc/hexanes to give the title compound as a light brown solid. 1H NMR (500 MHz, CD3OD): 1.71 (q, 1H, J=12 Hz), 2.56-2.61 (m, 1H), 3.11-3.18 (m, 1H), 3.36-3.40 (m, 1H), 3.48 (t, 1H, J=12 Hz), 3.88-3.94 (m, 4H), 4.30-4.35 (m, 1H), 4.53 (d, 1H, J=12 Hz), 7.14-7.23 (m, 2H), 7.26-7.30 (m, 1H), 7.88 (s, 1H). LC-MS: 399.04 (M+1). |
| 64.1 g |
With trifluoroacetic acid In water at 5 - 25℃; Inert atmosphere; |
|
|
With potassium fluoride; benzenesulfonic acid In dichloromethane; water at 20℃; Inert atmosphere; Large scale; |
B Step B: (2R,35,5R)-2-(2,5-Difluorophenyl)-5-[2- (methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine
Step B: (2R,3S,5R)-2-(2,5-Difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine Benzenesulfonic acid (32.95 kg, 271 mol) was dissolved in dichloromethane (1020 kg) under nitrogen. Then, 880 g of water was added such that the solution KF was 0.2%. Next, tert-butyl {(2R,3S,5R)-2-(2,5-difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-yl}carbamate (38.4 kg, 100 mol) was added in three equal portions over 30 min. The reaction was then aged overnight at room temperature. Next, water (733 kg) was added over 1 h and the reaction stirred rapidly for 1 h. The layers were then separated, discarding the resulting organics layer. To the aqueous layer was charged dichloromethane (510 kg) followed by triethylamine (22.4 kg, 592 mol). After agitation, the layers were separated and the aqueous extracted with dichloromethane (510 kg). The combined organics were washed with 7% aqueous NaHCO3 (2*410 kg) and 5% brine (386 kg). The organics were then dried with Na2SO4, filtered, and treated with activated carbon (6.2 kg of C-941). The carbon was filtered off and the filtrate was concentrated under vacuum to 154-193 L. This solution was then warmed to 30-35° C. to dissolve solids (additional dichloromethane may be added to dissolve solids). Next, iso-propylacetate (338 kg) was added and the solution stirred at room temperature for 1.5 h. Then, n-heptane (159 kg) was charged to the vessel drop-wise and stirred for 3 h. The slurry was then filtered and the cake washed with n-heptane. This wet cake was then recrystallized again by dissolving it into dichloromethane and adding iso-propylacetate and n-heptane as before, filtering, and washing with n-heptane. The solids were dried under vacuum at 40-50° C. overnight to give crystalline (2R,3S,5R)-2-(2,5-Difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine was washed with cold 2:1EtOAc/hexanes to give the title compound as an off-white solid. 1H NMR (500 MHz, CD3OD): 1.71 (q, 1H, J=12 Hz), 2.56-2.61 (m, 1H), 3.11-3.18 (m, 1H), 3.36-3.40 (m, 1H), 3.48 (t, 1H, J=12 Hz), 3.88-3.94 (m, 4H), 4.30-4.35 (m, 1H), 4.53 (d, 1H, J=12 Hz), 7.14-7.23 (m, 2H), 7.26-7.30 (m, 1H), 7.88 (s, 1H). LC-MS: 399.04 [M+1]. |
|
With potassium fluoride; benzenesulfonic acid In dichloromethane; water at 20℃; Inert atmosphere; Large scale; |
B Step B: (2R,3S,5R)-2-(2,5-Difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine
Step B: (2R,3S,5R)-2-(2,5-Difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine:_Benzenesulfonic acid (32.95 kg, 271 mol) was dissolved in dichloromethane (1020 kg) under nitrogen. Then, 880 g of water was added such that the solution KF was 0.2%. Next, tert-butyl {(2R,3S,5R)-2-(2,5-difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-yl}carbamate (38.4 kg, 100 mol) was added in three equal portions over 30 min. The reaction was then aged overnight at room temperature. Next, water (733 kg) was added over 1 h and the reaction stirred rapidly for 1 h. The layers were then separated, discarding the resulting organics layer. To the aqueous layer was charged dichloromethane (510 kg) followed by triethylamine (22.4 kg, 592 mol). After agitation, the layers were separated and the aqueous extracted with dichloromethane (510 kg). The combined organics were washed with 7% aqueous NaHCO3 (2×410 kg) and 5% brine (386 kg). The organics were then dried with Na2SO4, filtered, and treated with activated carbon (6.2 kg of C-941). The carbon was filtered off and the filtrate was concentrated under vacuum to 154-193 L. This solution was then warmed to 30-35° C. to dissolve solids (additional dichloromethane may be added to dissolve solids). Next, iso-propylacetate (338 kg) was added and the solution stirred at room temperature for 1.5 h. Then, n-heptane (159 kg) was charged to the vessel drop-wise and stirred for 3 h. The slurry was then filtered and the cake washed with n-heptane. This wet cake was then recrystallized again by dissolving it into dichloromethane and adding iso-propylacetate and n-heptane as before, filtering, and washing with n-heptane. The solids were dried under vacuum at 40-50° C. overnight to give crystalline (2R,3S,5R)-2-(2,5-Difluorophenyl)-5-[2-(methylsulfonyl)-2,6-dihydropyrrolo[3,4-c]pyrazol-5(4H)-yl]tetrahydro-2H-pyran-3-amine was washed with cold 2:1EtOAc/hexanes to give the title compound as an off-white solid. 1H NMR (500 MHz, CD3OD): 1.71 (q, 1H, J=12 Hz), 2.56-2.61 (m, 1H), 3.11-3.18 (m, 1H), 3.36-3.40 (m, 1H), 3.48 (t, 1H, J=12 Hz), 3.88-3.94 (m, 4H), 4.30-4.35 (m, 1H), 4.53 (d, 1H, J=12 Hz), 7.14-7.23 (m, 2H), 7.26-7.30 (m, 1H), 7.88 (s, 1H). LC-MS: 399.04 [M+1]. |
|
With trifluoroacetic acid In N,N-dimethyl acetamide; water at 0 - 20℃; |
Boc Deprotection of Formula Ta
A reactor was charged with 2.5 X (by volume) of trifluoroacetic acid. The batch was cooled to 5-10 °C. The reactor was then charged with 0.4 X (by volume) water. The batch was cooled to 0-5 °C. The reactor was then charged with 1 equivalent (1 kg) of the compound ofFormula Ia over 0.5-lh while maintaining the temperature between 0 -5°C. The reactor was then charged with 0.5 X (by volume) trifluoroacetic acid to reactor while maintaining the temperature between 0-5°C. The batch was then heated between 15-20°C and aged for 2-2.5 h. The batch was then cooled to between 5-10°C. A crystallizer was charged with water 5.0 X (by volume) and 0.1 X (by volume) of ammonia water and adjusted to between 3-13°C. To generate a seed bed, Compound I seed (lwt% vs Ia) was added and the temperature as adjusted to between 3-13°C. A solution of ammonia water 3.8 X (by volume) and of the compound of Formula Ta was added simultaneously to the seed bed over 2.5 - 3.5 hours while maintainingtemperature at 3-13°C and pH 9-1O. The batch was aged for at least 30 minutes and then filtered. The resulting crystals were washed with 3.OX (by volume) water at 3 - 13°C twice and vacuum dried at E 50°C to afford the compound of formula I. |
| 6.73g |
With sulfuric acid In N,N-dimethyl acetamide; water at 20℃; for 18.5h; Inert atmosphere; |
1.2
2) The aminated product will be reduced (10.35g) andDMAc (31 mL) and water (41.4 mL)Into a 200 mL three neck jacketed flask equipped with overhead stirrer, N2 inlet and thermocouple,And the resulting slurry was stirred at Ti = 20 ° C.H2SO4 (12.2 mL; 12 equivalents) and slowly added over 3.5 hoursH2O (20.7 mL).The resulting slurry was aged for 15 hours.The solution is then cooled to Ti = 0-5 ° C. Add NH4OH untilThe pH of the supernatant was 10.2. Cool and filter the slurry. The wet cake was replaced with cold H 2 O (17.5 mL)The slurry was washed then with H2O (17.5 mL). The recovered solids were dried to give 6.73 g of the ologrellate solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping