Alternatived Products of [ 1221509-80-3 ]
Product Details of [ 1221509-80-3 ]
| CAS No. : | 1221509-80-3 |
MDL No. : | MFCD32667034 |
| Formula : |
C42H27N3O3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | GDGIVFCQFWIYBY-UHFFFAOYSA-N |
| M.W : |
621.68
|
Pubchem ID : | 145926288 |
| Synonyms : |
|
Safety of [ 1221509-80-3 ]
Application In Synthesis of [ 1221509-80-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1221509-80-3 ]
- 1
-
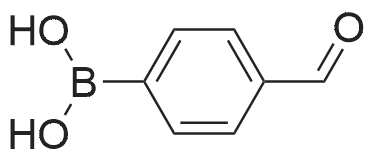 [ 87199-17-5 ]
[ 87199-17-5 ]

-
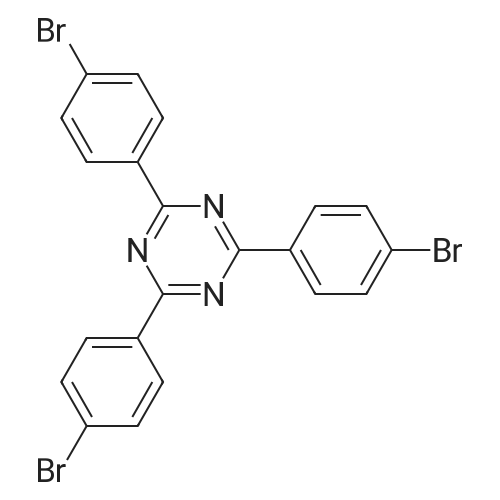 [ 30363-03-2 ]
[ 30363-03-2 ]

-
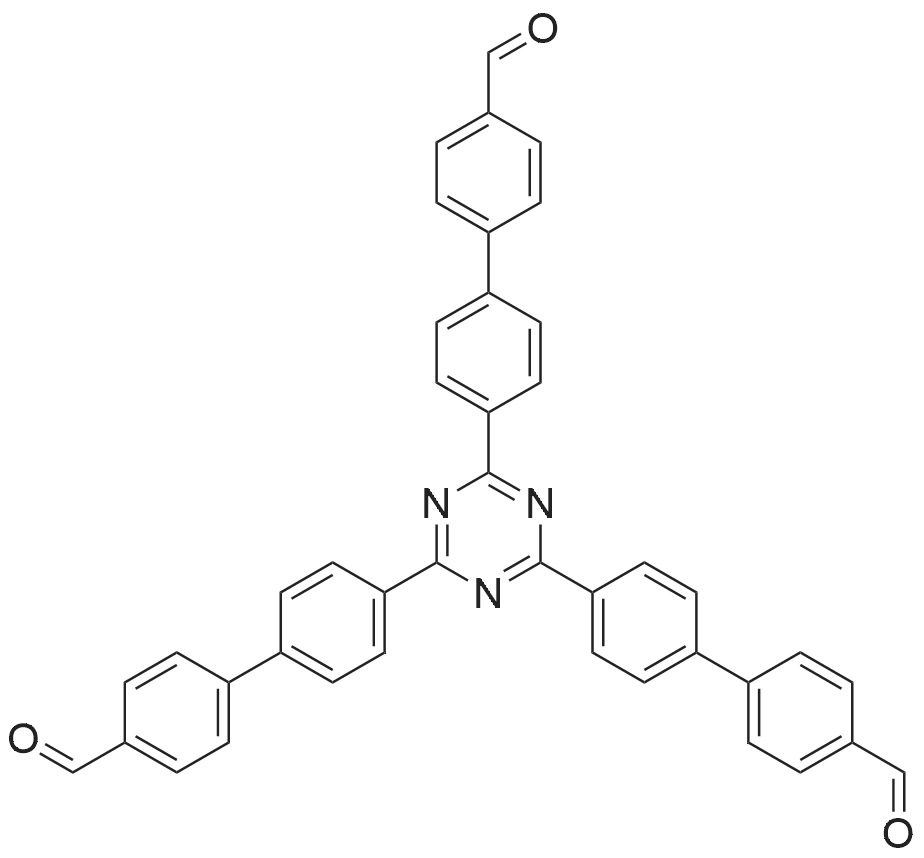 [ 1221509-80-3 ]
[ 1221509-80-3 ]
- 2
-
 [ 1221509-80-3 ]
[ 1221509-80-3 ]

-
C33H49NO4
[ No CAS ]

-
C141H168N6O12
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 31% |
With sodium hydroxide In tetrahydrofuran; ethanol |
2.1 (1) The luminescent liquid crystal material is prepared by using the intermediate 1 (Y1-1) and the intermediate 2 (Y2-1) prepared in the first embodiment as a substrate, and the specific preparation steps are as follows:
In a 100 mL single-necked flask, a magnet was charged, and 186 mg (0.30 mmol) of Intermediate 1 (Y1-1), 566 mg (1.08 mmol) of Intermediate 2 (Y2-1), and poured into anhydrous ethanol (EtOH) 40 mL, tetrahydrofuran ( THF) 15mL promoted the dissolution of the reactants; stirred at room temperature, then weighed 0.35g of NaOH solids, slowly added to the flask several times, then covered with a stopper, and tied with a syringe needle to maintain pressure balance; monitoring with TLC board After the reaction is completed, 10% by weight of dilute hydrochloric acid is added to adjust the pH value, and neutrality can be detected by using pH test paper; then the solvent is spin-dried, ultrasonically dissolved by adding appropriate amount of chloroform, transferred to a separating funnel, and water turbid layering is added. After the extraction, the organic layer was washed with water three times, and the aqueous layer was combined and then extracted with chloroform. The organic layer was combined and dried over anhydrous MgSO 4 to afford the crude product. The crude product was dissolved in chloroform and purified by column chromatography. Further, it was recrystallized to obtain 200 mg of a luminescent liquid crystal material, which was designated as Z1-1; Z1-1 was a yellow-white solid, yield 31%. |
- 3
-
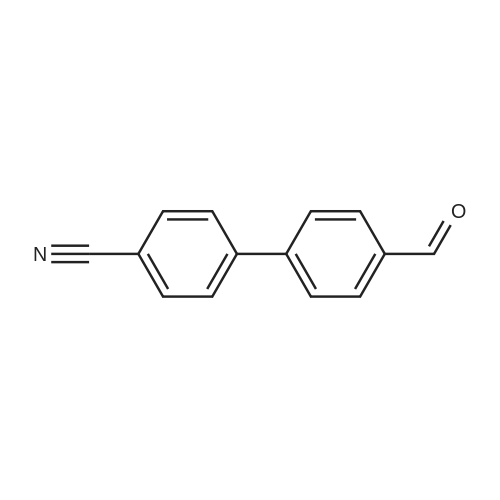 [ 50670-55-8 ]
[ 50670-55-8 ]

-
 [ 1221509-80-3 ]
[ 1221509-80-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 18.3% |
With trifluorormethanesulfonic acid In chloroform at 20℃; for 13h; Inert atmosphere; Cooling with ice; Reflux; |
1.1 (1) Preparation of Intermediate 1 in which Ar is prepared, and the preparation route is as follows:
Take a 50 mL two-necked flask, put in a magnet, add 2.0 g (9.65 mmol) of the reactant X1, start stirring, vacuum and pass nitrogen three times, dissolve with 20 mL of chloroform, and use a constant pressure funnel in an ice bath. 2.56 mL (28.95 mmol) of trifluoromethanesulfonic acid was slowly added and stirred for 1 h; after stirring to room temperature, stirring was continued, and then the reaction was heated under reflux for 12 h, and the progress of the reaction was monitored by TLC plate; after completion of the reaction, the solution was quenched with NaHCO3 saturated solution. Fluoromethanesulfonic acid, the mixture was filtered, and the obtained solid was washed three times with n-hexane, water and dichloromethane to give a yellow-brown powder (the filtrate and the washings were collected, and the obtained organic layer was extracted with dichloromethane and then dried. Most of them are lost products, but it is difficult to purify. After drying, they are recrystallized to obtain 1.1 g of Intermediate 1, which is named Y1-1, and the yield is 18.3%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







