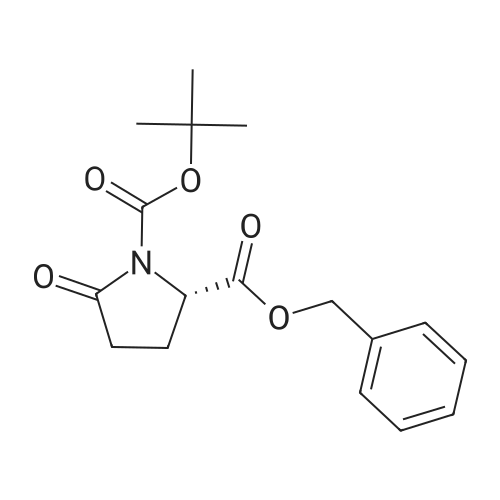
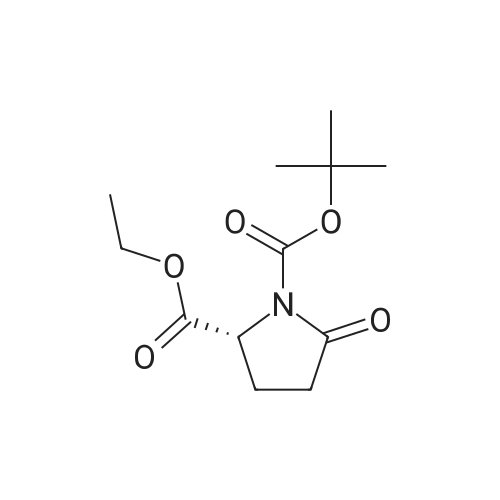
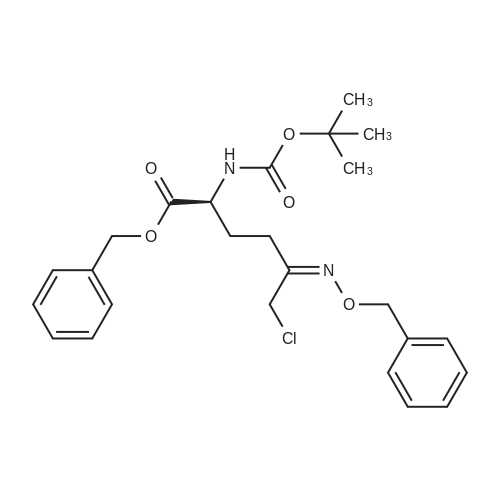
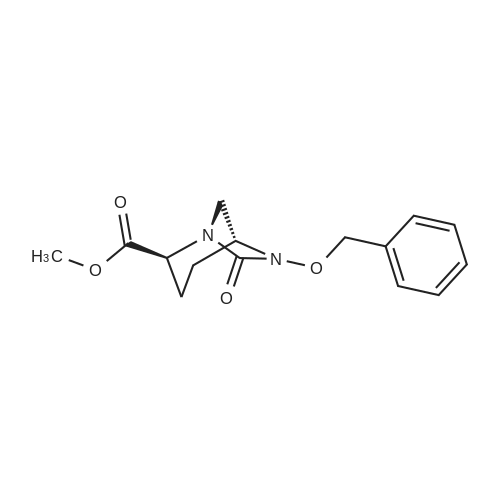
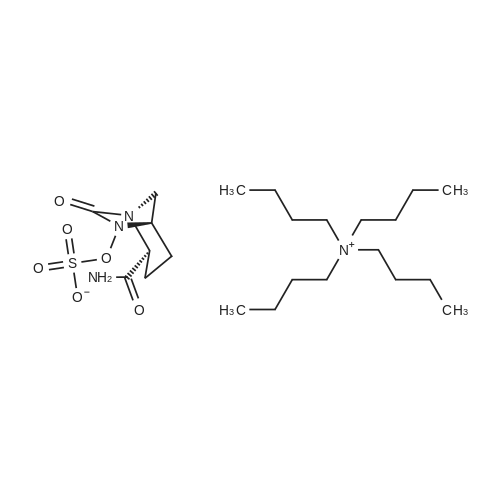
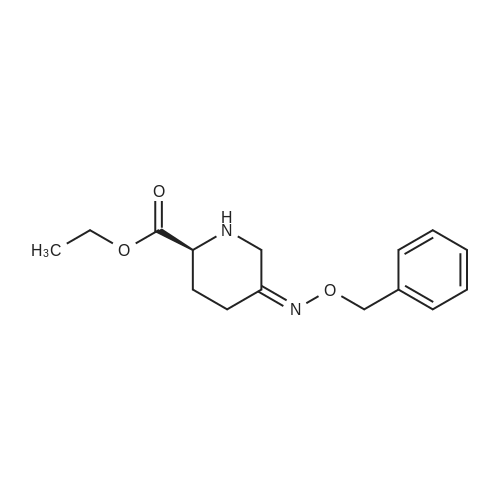
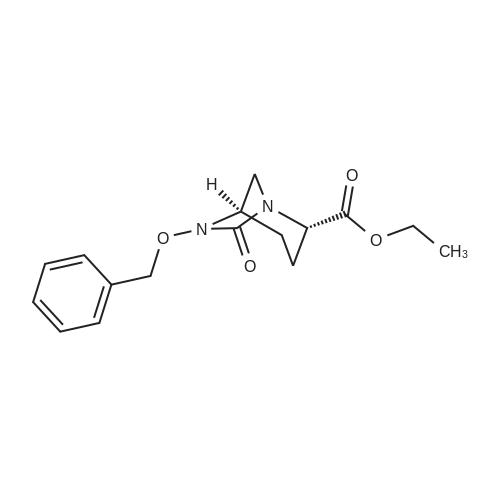
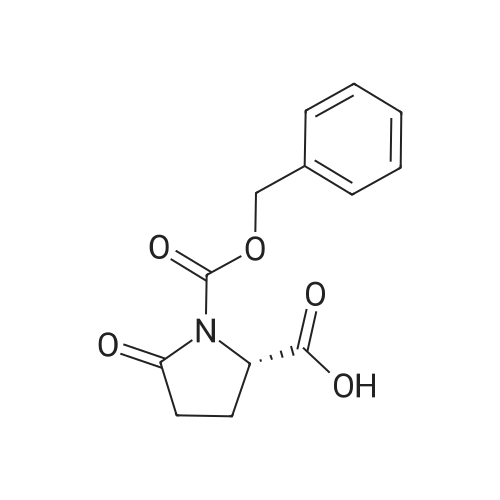
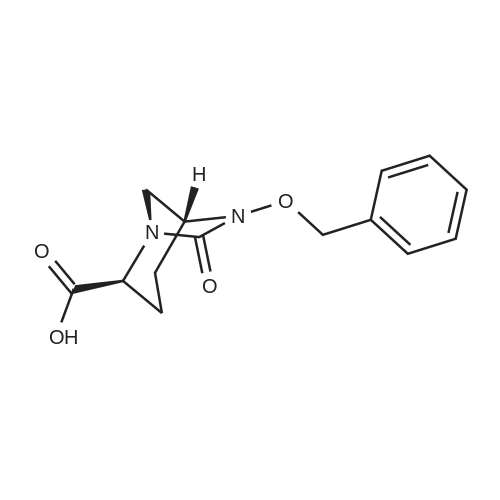
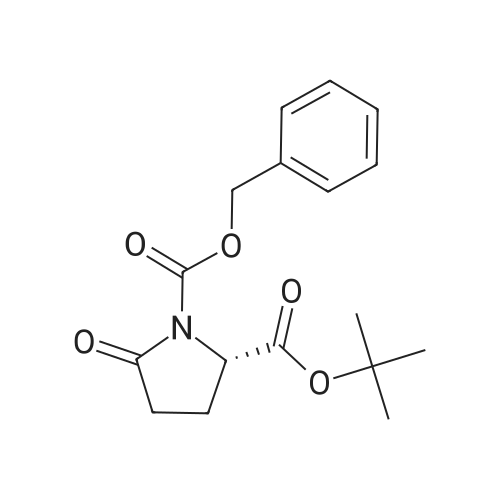
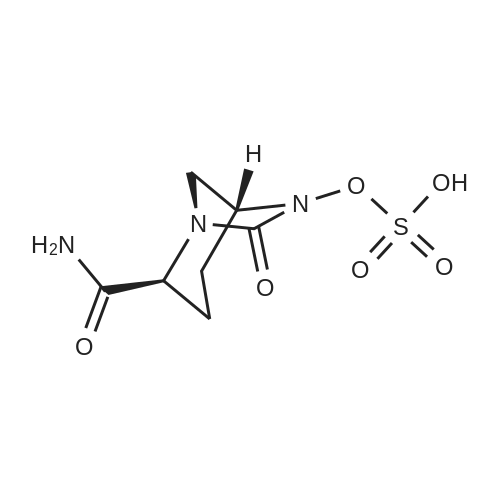
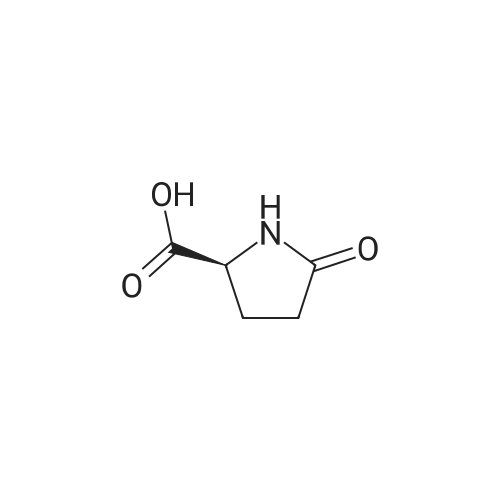
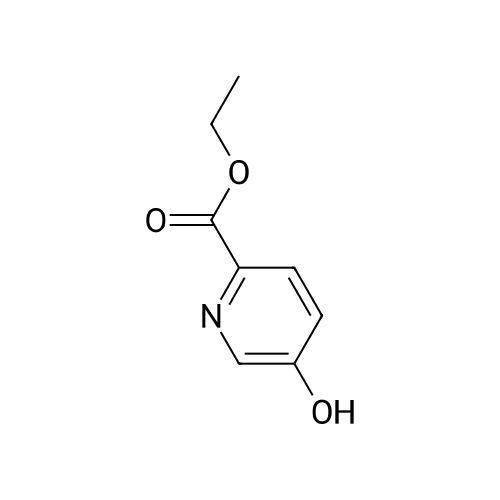
| 91.5% |
With sodium 2-ethylhexanoic acid; In ethanol; at 20 - 25℃; for 3h; |
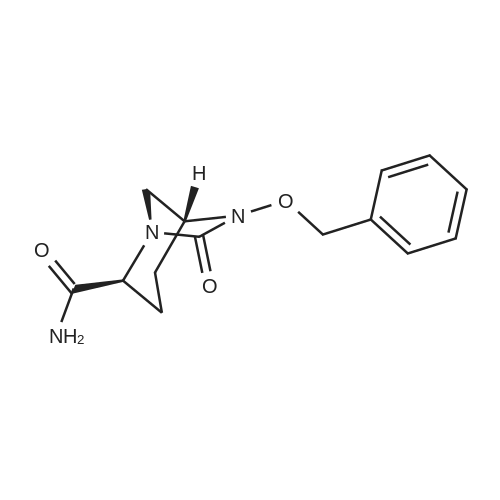
Stirring,Add 260 g of ethanol (2 wt% water) to a 500 ml flask of the thermometer.50.6 g (0.1 mol)[(2S,5R)-2-carbamoyl-7-oxo-1,6-diazacyclo[3.2.1]octane-6-yl]oxy}sulfonyltetra-n-butylammonium salt Stir at 20-25 C with stirring.33.2 grams (0.2 moles) in advanceSodium isooctanoateSoluble in 280.0 grams of ethanol,Prepared as a solution.The solution was added dropwise to the above system at 20-25 C to precipitate a white solid.After the dropwise addition was completed, the mixture was stirred at 20 to 25 C for 3 hours.Filter by suction and wash with 100.0 g of ethanol.Obtained 26.2 g of acarbamate (I) with a liquid phase purity of 99.9%.The yield was 91.5%. |
| 90% |
With sodium 2-ethylhexanoate; In ethanol; water; at 20 - 25℃; for 24h; |
Sodium 2-ethylhexanoate (196.8) was added to a reaction flask, stirred, and a solution of ethanol (2000 g) and water (50 g) were added.K (500g) was dissolved in ethanol (2000g), added dropwise to the reaction flask, stirred at a temperature of 20 C ~ 25 C for 24 hours, cooled to 0 C filtration,Drying at room temperature at 25 C under vacuum gave a yield of 90%, a purity of 99.8%, and a single impurity <0.1% of avivatan sodium (255 g). |
| 84% |
With sodium 2-ethylhexanoic acid; In ethanol; water; for 12h; |
The solution of tetrabutylammonium bromide (100.0 g, 197.4 mmol) in ethanol (1000 ml) was added dropwise over 1 hTo a solution of sodium 2-ethylhexanoate (38.1 g, 229.2 mmol) in ethanol (950 ml) and water (50 ml) was stirred the reaction for 14 h. Cool to 0-5 C, hold for 1 h, filter and wash with ethanol (100 ml). Drying under reduced pressure at 35 C for 6h afforded the compound of formula I as crystals (47.6g, yield: 84.0%). |
| 83% |
With sodium 2-ethylhexanoic acid; In ethanol; at 35℃; for 2h; |
To a 100 ml round bottom flask equipped with magnetic stirrer was charged tetrabutyl ammonium salt of (2S, 5R)-2-carboxamido-6-sulfooxy-7-oxo-l,6-diaza-bicyclo[3.2.1]octane( 5.5 gm, 0.0108 mol) followed by ethanol (28 ml) to provide a clear solution under stirring at about 35C. To the reaction mixture was added a solution of sodium 2-ethyl hexanoate (3.6 gm, 0.021 mol) dissolved in ethanol (28 ml) in one lot under stirring to provide precipitation. The suspension was stirred for additional 2 hours to effect complete precipitation at about 35C. The reaction mixture was filtered under suction and the wet cake was washed with acetone (30 ml x 2). The wet cake was dried at 40C under vacuum to provide sodium salt of (2S, 5R)-2-carboxamido-6-sulfooxy-7-oxo-l,6-diaza-bicyclo[3.2.1]octane as a white solid in 2.6 gm quantity in 83% yield. Analysis H NMR (DMSO-d6) 7.39 (s, 1H), 7.24 (s, 1H), 3.98 (s, 1H), 3.68 (d, 1H), 3.02 (d, 1H), 2.92 (d, 1H), 2.00- 2.10 (m, 1H), 2.80-2.90 (m, 1H), 1.55-1.70 (m, 2H). MS (ES-) C7H10N3O6SNa = 264.0 (M-l) as a free sulfonic acid; Purity: 97.98% as determined by HPLC Specific rotation: [a]25D - 49.37 (c 0.5%, water) |
| 21 g |
|
20g of compound I was added into a 1000ml reaction flask, 400ml of tetrahydrofuran was added and 35g of diisopropylEthyl ethylamine, the control temperature -10 ~ 10 dropwise 11.3g triphosgene. TLC detection until the reaction is completed. Rise to room temperature and add dropwise hydrogenLiquefied lithium saturated solution, control the feed solution PH value of about 10 stirring for 1 hour, after the completion of TLC test reaction with hydrochloric acid to the reaction solution was adjustedAcidic, extracted with 300 ml of dichloromethane. The organic layer was dried over anhydrous sodium sulfate, filtered off with suction, washed, and 7.7 g of triethylamine and was added8.2g n-butyl chloroformate, the control temperature -25 ~ 5 37g concentrated ammonia solution (mass fraction of 25% -28%), TLC detection of anti-After the completion of the reaction layer should be layered. Under nitrogen atmosphere, 2 g of palladium hydroxide carbon and 10.9 g of ammonium formate were added to the reaction mixture to controlThe reaction was stirred at 30-40 C for 5 hours. Insoluble material was filtered off after completion of the TLC test and 2.2 g of triethylamine was added to the filtrateAnd 7.5g trimethylamine trioxide, the reaction was stirred at room temperature for 4 hours, 24.5g of tetrabutylammonium acetate was added and the reaction was stirred for 1 hour. StaticThe layers were separated and washed to obtain a reaction solution. Then, a solution of sodium isooctanoate in ethanol (18 g of sodium isooctanoate + 180 mlEthanol), cooling crystallization, suction filtration, washing and drying to obtain compound VI9.21g, yield: 60.9%, purity: 99.9%. |
| 1.1 g |
With sodium 2-ethylhexanoic acid; In ethanol; at 20 - 35℃; for 18h; |
General procedure: Preparation of a compound of Formula (I-e) (0144) Formula (I-e) (0145) The O-sulfonation reagent was prepared according to the procedure given in Example g triethylamine (4.41 gm, 0.0436 mol) and chloro sulfonic acid (2.55 gm, 0.0218 mol). (0146) Formula (II- e) (0147) (0148) The compound of Formula (Il-e) (2.00 gm, 0.0109 mol) was reacted with the O-sulfonation reagent in presence of triethyl amine (3.31 gm, 0.0327 mol) in dichloro methane to obtain 4.2 gm of tetra-butyl ammonium salt of Formula (Ill-e). (0149) (0150) The compound of Formula (Ill-e) was analyzed as follows. (0151) Analysis: (0152) IH NMR (CDC13): 6.66 (br s, IH), 5.59 (br s, IH), 4 34 (br s, IH), 3 .91 (d, IH), 3.28-3.38 (m, 9H), 3.14 (d, IH), 2.36-2.41 (m, IH), 2.13-2.15 (m, IH), 1.87-1.94 (m, 2H), 1.62-170 (m, 8H), 1.36-1.49 (m, 8H), 1.00 (t, 12H). The tetra-butyl ammonium salt of Formula (Ill-e) (4.1 gm, 0.0081 mol) was dissolved in ethanol (16.4 ml) to obtain a clear solution. To the clear solution, was charged ethanolic solution of sodium-2-ethyl-hexanoate (2.68 gm, 0.016 mol; dissolved in 16.4 ml ethanol) at a temperature range within 20 - 35C, under stirring. The reaction mixture was stirred for 18 hours and the suspension so obtained was filtered under suction. The wet cake was washed with acetone (10 ml X 2), and dried below 40C under vacuum to provide 1.1 gm of the compound of Formula (I-e). (0153) Analysis: (0154) 1H NMR (DMSO-d6): 7.44 (br s, 1H), 7.29 (br s, 1H), 3.97 (br s, 1H), 3.67 (d, 1H), 3.01 (d, 1H), 2.91 (d, 1H), 2.01-2.10 (m, 1H), 1.80-1.90 (m, 1H), 1.57-1.68 (m, 2H). (0155) HPC Purity: 99.09% |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping