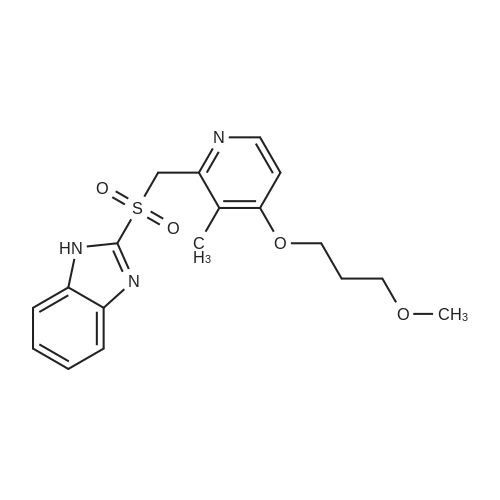
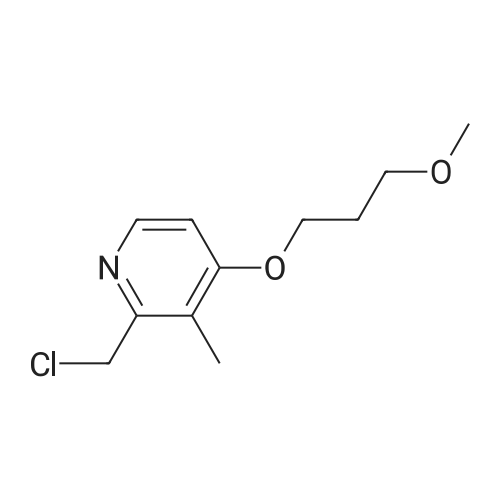
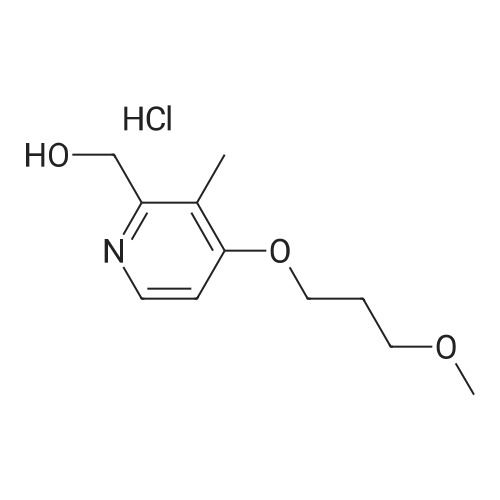
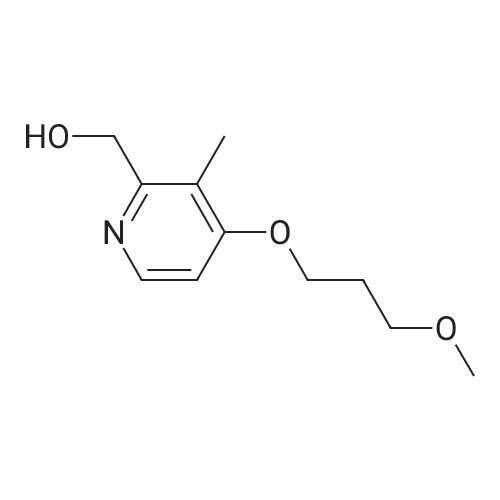
| 97% |
With sodium hydroxide; In water; at 4 - 5℃; |
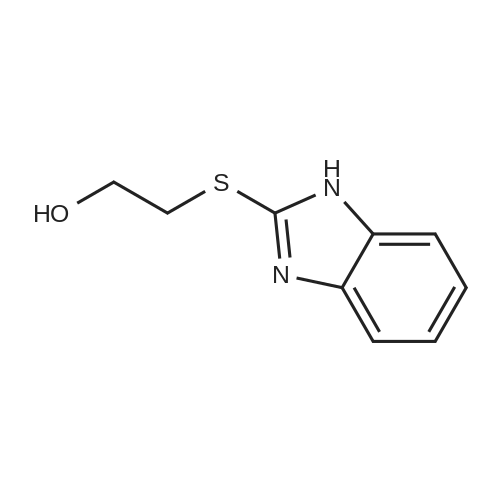
Sodium hydroxide (5.62 g) was dissolved in DM water (200 ml) and cooled to [4-5C.] Rabeprazole (50 g) was added and mixture stirred to obtain a clear solution. The solution was treated with 2 g of carbon DC-enoanticromos for 30 min at [5-10C.] Carbon was removed by filtration and residue washed with DM water (2x25 ml). The contents were [LYOPHILIZED] using standard method. Rabeprazole sodium was obtained as a white powder. |
| 94.9% |
With sodium hydroxide; In methanol; at 25 - 35℃; |
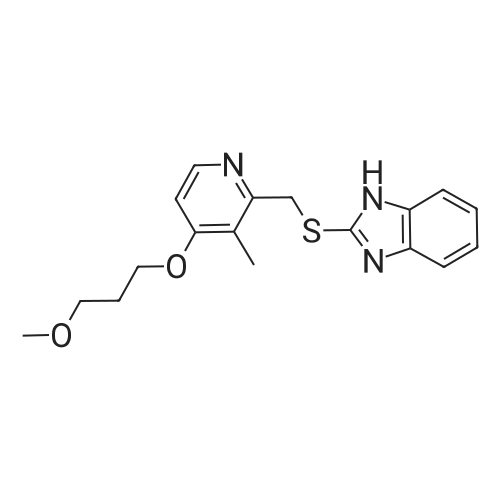
2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1H-benzimidazole (obtained as per reference example 1) (50.0 grams, 0.139 moles) was dissolved in a mixture of sodium hydroxide (7.5 grams, 0.187 moles) and methanol (100.0 ml), and the solution was stirred at ambient temperature 25-35 C. The reaction solution is filtered through hi-flow and washed with methanol (50.0 ml). Then, the solvent of the filtrate was distilled off under reduced pressure. The reaction mass was cooled to ambient temperature, and petroleum ether (400.0 ml) was then added to the residual mass, which was then stirred at 25-30 C for about 1-2 hours. The precipitated solid was filtered and washed with petroleum ether (100.0 ml) and dried at 50-60C for 12 hours to afford the desired amorphous form of rabeprazole sodium (Weight: 50.4 grams, 94.9%). |
| 94.9% |
With sodium hydroxide; In methanol; at 25 - 35℃; for 1h; |
2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1H-benzimidazole (obtained by reference example 1) (50.0 grams, 0.139 moles) was dissolved in a mixture of sodium hydroxide (7.5 grams, 0.187 moles) and methanol (100.0 ml), and the resulted solution was stirred at ambient temperature 25-35C for about one hour. The reaction solution was filtered through hi-flow and washed with methanol (50.0 ml). Then the solvent of the filtrate was distilled off under reduced pressure. The reaction mass is cooled to ambient temperature, to which toluene (200.0 ml) was added. The residual mass was then refluxed for about 2-6 hours. After cooling the reaction solution, the precipitated solid was filtered, washed with toluene (100.0 ml) and dried at 90-100C for 12 hours to afford the desired form Z ofrabeprazole sodium (Weight: 50.4 grams, 94.9%). |
| 94.9% |
With sodium hydroxide; In methanol; at 25 - 35℃; |
2-[[[4-(3-methoxypropoxy)-3-melhyl-2-pyridinyl]-methyl] sulfinyl]-1H-benzimidazole (obtained by reference example 1) (50.0 grams, 0.139 moles) was dissolved in a mixture of sodium hydroxide (7.5 grams, 0.187 moles) and methanol (100.0 ml) and stirred at ambient temperature 25-35C. The reaction solution was filtered through hi-flow and washed with methanol (50-0 ml). Then the solvent of the filtrate was distilled off under reduced pressure. The reaction mass was cooled to ambient temperature. Dichloromethane (100.0 ml) was added to the reaction mixture, which was then distilled to remove traces of methanol. Dichloromethane (50.0 ml) and petroleum ether (100.0 ml) was then added to the residual mass, which was then stirred at 25-30 C for about 6-8 hours. Additional petroleum ether (150 ml) was added to the reaction mixture, which was stirred at 25-30 C for 1-2 hours. The precipitated solid was filtered and washed with petroleum ether (100.0 ml) and dried at 50-60C for 12 hours to afford the desired form X of rabeprazole sodium (Weight: 50.4 grams, 94.9%). |
| 94.9% |
With sodium hydroxide; In methanol; at 25 - 35℃; for 1h;Purification / work up; |
2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1H-benzimidazole (obtained by reference example 1) (50.0 grams, 0.139 moles) was dissolved in a mixture of sodium hydroxide (7.5 grams, 0.187 moles) and methanol (100.0 ml), and the resulted solution was stirred at ambient temperature 25-35 C. for about one hour. The reaction solution was filtered through hi-flow and washed with methanol (50.0 ml). Then the solvent of the filtrate was distilled off under reduced pressure. The reaction mass is cooled to ambient temperature, to which toluene (200.0 ml) was added. The residual mass was then refluxed for about 2-6 hours. After cooling the reaction solution, the precipitated solid was filtered, washed with toluene (100.0 ml) and dried at 90-100 C. for 12 hours to afford the desired form Z of rabeprazole sodium (Weight: 50.4 grams, 94.9%). |
| 94.9% |
|
Example 1; Preparation Of Crystalline Form-X Of Rabeprazole Sodium 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl] sulfinyl]-lH- benzimidazole (obtained as per reference example) (50.0 grams, 0.139 moles) is dissolved in a mixture of sodium hydroxide (7.5 grams, 0.187 moles) and methanol (100.0 ml) and stirred at ambient temperature. The reaction solution is filtered through hi-flow and washed with methanol (50.0 ml). Methanol from the filtrate is distilled off under high vacuum. The reaction mass is cooled to ambient temperature followed by addition of dichloromethane (100.0 ml) accompanied by distillation to remove traces of methanol. Dichloromethane (50.0 ml) and petroleum ether (100.0 ml) is then added to the residual mass, which is then stirred at 25-30C for about 6-8 hours. The solid that is obtained further diluted with petroleum ether (150 ml) and stirred at 25-30C for about 6-8 hours. The precipitated solid is filtered and washed with petroleum ether (100.0 ml) and dried at 50-60C for 12 hours to afford the desired Form X of Rabeprazole sodium (Weight: 50.4 grams, 94.9%). The X-ray Diffraction Pattern, Differential Scanning Calorimetry thermogram of Form X of Rabeprazole sodium obtained in above example is in accordance with Figure 1 and 2 respectively. |
| 94% |
With methanol; sodium; at 20 - 30℃;Inert atmosphere; |
1.73 g (0.075 mol) of sodium metal was dissolved in 120 ml of methanol at room temperature under nitrogen atmosphere. 27 g (0.075 mol) of 2-[[[4-(3-methoxy propoxy)- 3 -methyl-2-pyridinyl)] methyl] sulfinyl]-lH-benzimidazole was added to the solution at room temperature. The obtained mixture was stirred for 15 minutes to get clear solution. The clear reaction mass was stirred at 300C for one hour. 2.7 g of activated charcoal was added & stirred for one hour (30+/-2 0C). Filtered the reaction mixture, the clear filtrate was concentrated under reduced pressure to obtain 29 g of crude title compound. The above crude solid was crystallized from a mixture of 87 ml methylene dichloride and 290 ml of t-butyl methyl ether in a nitrogen atmosphere to obtain 27 g (94%) of title compound |
| 93% |
With sodium hydroxide; In methanol; at 20℃; for 1h; |
1L of three-necked flask, was added 4g (0.1mol) in 100ml of methanol solution of sodium hydroxide, followed by addition of 36g of rabeprazole (0.1 mol), the reaction was stirred for 1h at room temperature to give a clear solution.Filtered and the filtrate rotary crude done.Room temperature using 150ml The crude product was dissolved in dichloromethane, and then 400ml of n-heptane was slowly added dropwise, the solution gradually became cloudy, and then stirred at room temperature after the addition was complete 1h.Filtered off with suction, the filter cake was rinsed with n-heptane alkoxy filter cake in a vacuum oven, 40-45 blast drying 12h, weighed to obtain rabeprazole sodium finished 35.62g (0.093mol), 93% yield. |
| 91.1% |
With sodium hydroxide; In methanol; at 25 - 35℃; |
2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1H-benzimidazole (obtained as per reference example 1) (750.0 grams, 2.089 moles) was dissolved in a mixture of sodium hydroxide (112.5 grams, 2.8125 moles) and methanol (1500.0 ml), and the resulting solution was stirred at ambient temperature 25-35C. The reaction solution was filtered through hi-flow and washed with methanol (750.0 ml). The solvent of the filtrate was distilled off completely under reduced pressure. The reaction mass was cooled to ambient temperature, and dichloromethane (1500.0 ml) was added to the reaction mass. The solvent was distilled again to remove traces of methanol. The reaction mass was cooled to ambient temperature, and n-butanol (375.0 ml) and tertiary butyl methyl ether (6.0 L) was added to the residual mass which is stirred at 25-30C for 6-8 hours. The reaction mixture was further cooled to 5-15 C and then stirred for another 3-5 hours. The solid then precipitated was filtered and washed with tertiary butyl methyl ether (1500.0 ml) and dried at 50-60C for 7 hours to afford the desired crystalline Form Y of rabeprazole sodium (Weight: 725.0 grams, 91.1%). |
| 91.1 - 94.9% |
With sodium hydroxide; In methanol; at 25 - 35℃; for 1h;Purification / work up; |
2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1H-benzimidazole (obtained as per reference example 1) (50.0 grams, 0.139 moles) was dissolved in a mixture of sodium hydroxide (7.5 grams, 0.187 moles) and methanol (100.0 ml), and the solution was stirred at ambient temperature 25-35 C. The reaction solution is filtered through hi-flow and washed with methanol (50.0 ml). Then, the solvent of the filtrate was distilled off under reduced pressure. The reaction mass was cooled to ambient temperature, and petroleum ether (400.0 ml) was then added to the residual mass, which was then stirred at 25-30 C. for about 1-2 hours. The precipitated solid was filtered and washed with petroleum ether (100.0 ml) and dried at 50-60 C. for 12 hours to afford the desired amorphous form of rabeprazole sodium (Weight: 50.4 grams, 94.9%). REFERENCE EXAMPLE 3 [0052] Preparation of Crystalline form X of 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1H-benzimidazole sodium (Rabeprazole Sodium) [0053] 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1H-benzimidazole (obtained by reference example 1) (50.0 grams, 0.139 moles) was dissolved in a mixture of sodium hydroxide (7.5 grams, 0.187 moles) and methanol (100.0 ml) and stirred at ambient temperature 25-35 C. The reaction solution was filtered through hi-flow and washed with methanol (50.0 ml). Then the solvent of the filtrate was distilled off under reduced pressure. The reaction mass was cooled to ambient temperature. Dichloromethane (100.0 ml) was added to the reaction mixture, which was then distilled to remove traces of methanol. Dichloromethane (50.0 ml) and petroleum ether (100.0 ml) was then added to the residual mass, which was then stirred at 25-30 C. for about 6-8 hours. Additional petroleum ether (150 ml) was added to the reaction mixture, which was stirred at 25-30 C. for 1-2 hours. The precipitated solid was filtered and washed with petroleum ether (100.0 ml) and dried at 50-60 C. for 12 hours to afford the desired form X of rabeprazole sodium (Weight: 50.4 grams, 94.9%). REFERENCE EXAMPLE 4 [0054] Preparation of Crystalline form-Y of 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1H-benzimidazole sodium (Rabeprazole Sodium) [0055] 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1H-benzimidazole (obtained as per reference example 1) (750.0 grams, 2.089 moles) was dissolved in a mixture of sodium hydroxide (112.5 grams, 2.8125 moles) and methanol (1500.0 ml), and the resulting solution was stirred at ambient temperature 25-35 C. The reaction solution was filtered through hi-flow and washed with methanol (750.0 ml). The solvent of the filtrate was distilled off completely under reduced pressure. The reaction mass was cooled to ambient temperature, and dichloromethane (1500.0 ml) was added to the reaction mass. The solvent was distilled again to remove traces of methanol. The reaction mass was cooled to ambient temperature, and n-butanol (375.0 ml) and tertiary butyl methyl ether (6.0 L) was added to the residual mass which is stirred at 25-30 C. for 6-8 hours. The reaction mixture was further cooled to 5-15 C. and then stirred for another 3-5 hours. The solid then precipitated was filtered and washed with tertiary butyl methyl ether (1500.0 ml) and dried at 50-60 C. for 7 hours to afford the desired crystalline Form Y of rabeprazole sodium (Weight: 725.0 grams, 91.1%). |
| 91.1% |
|
Example 2; Preparation Of Crystalline Form-Y Of Rabeprazole Sodium 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl] sulfinyl]-1H- benzimidazole (obtained as per reference example) (750.0 grams, 2.089 moles) is dissolved in a mixture of sodium hydroxide (112.5 grams, 2. 8125 moles) and methanol (1500.0 ml) and stirred at ambient temperature. The reaction solution is filtered through hi-flow and washed with methanol (750.0 ml). Methanol from the filtrate is distilled off under high vacuum. The reaction mass is cooled to ambient temperature followed by addition of dichloromethane (1500.0 ml) accompanied by distillation to remove traces of methanol. The reaction mass is cooled to ambient temperature and n-butanol (375.0 ml) and tertiary butyl methyl ether (6.0 lit) is added to the residual mass which is stirred at 25- 30C for 6-8 hours. The reaction mixture is further cooled to 5-15C and then stirred for another 3-5 hours. The solid is thus obtained is filtered and washed with tertiary butyl methyl ether (1500.0 ml) and dried at 50-60C for 7 hours to afford the desired crystalline Form Y of Rabeprazole sodium (Weight: 725.0 grams 91. 1%). The X-ray Diffraction Pattern, Differential Scanning Calorimetry thermogram of Form Y of Rabeprazole sodium obtained in the example is in accordance with Figure 3 and 4 respectively. |
| 90.3% |
With sodium hydroxide; In methanol;Product distribution / selectivity; |
Example 1 2-[{4-(3-Methoxypropoxy)-3-methylpyridin-2-yl}methylsulfinyl]-1H-benzimidazole (4.97 g (13.8 mmol)) was dissolved in methanol (5 ml). Sodium hydroxide (0.559 g (13.7 mmol)) was then put into the methanol solution, and after dissolution had been confirmed, dipropyl ether (500 ml) was added slowly, whereby 2-[{4-(3-methoxypropoxy)-3-methylpyridin-2-yl}methylsulfinyl]-1H-benzimidazole sodium salt (4.77 g) was obtained through precipitation (yield 90.3%). |
| 90% |
|
Example- 1 Preparation of amorphous Rabeprazole sodium3.03 gm of freshly cut clean sodium metal was dissolved in 500 ml of tert. butyl alcohol under nitrogen atmosphere by refluxing to give a clear solution which was cooled to about 25-300C. Rabeprazole (50 gm) was added to it at about 300C and stirred for about one hour to give an orange brown solution. The <n="7"/>reaction mass was treated with about 2.5 gm of activated charcoal and stirred at 27-300C for about one hour. The reaction mass was filtered using hyio bed and lambdavashed with about 50 ml of tert. butyl alcohol. The filtered solution was distilled under vacuum at 40-500C to remove lert.butyl alcohol, high vacuum was applied for about 30 min to remove traces of lert. butyl alcohol. 750 ml of diisopropyl ether were added to the viscous mass, and stirred under nitrogen at 27-300C for about one hour. Off-white, amorphous powder of rabeprazole-Na salt was obtained, which was filtered under nitrogen and washed with 50 ml of diisopropyl ether. The product was dried under vacuum at 65-700C for about 48 hours to give amorphous rabeprazole sodium. Wt. of rabeprazole sodium = 47 gms % yield = 90 % purity > 99 % % water by ICF. < 4 % |
| 83 - 85% |
With sodium hydroxide; In water;Product distribution / selectivity; |
Example 1:Isolation of Rabeprazole sodium using ethyl acetate as solvent:Rabeprazole (50 gm) was dissolved in solution of sodium hydroxide (5.39 gm ) in demineralized water (162.5 ml). The solution was extracted with dichloromethane (100 ml) twice. The aqueous layer was then treated with neutral activated charcoal (1 gm) for 30 minutes at 28 to 35C. Carbon was removed by filtration and the residue washed with demineralized water (12.5 ml). Ethyl alcohol (50 ml) was added to the clear filtrate. The solution was concentrated to thick mass under vacuum at 40 - 45C. The thick mass was dissolved in ethyl alcohol (100 ml) and was again concentrated to a thick mass under vacuum at 40 - 45C. The residue was then dissolved in ethyl acetate (100 ml) and the solution was concentrated to a thick oily mass under vacuum. The residue thus obtained was dissolved in ethyl acetate (100 ml) and the solution was added slowly over a period of 20 to 30 minutes to diisopropyl ether (500 ml). The slurry thus obtained was stirred for 60 minutes at 28 to 35.. The solid was filtered and washed with diisopropyl ether. Then the solid was dried at 45 - 50 to get Rabeprazole sodium as a white amorphous solid (powder X-ray diffraction of the product does not show any sharp peak, shows only the base line), with mean particle diameter ranging between 10 to 50mum. Yield: 45 gm (85 %), Assay: 99.5 % (by HPLC). IR Spectra (KBr, cm'1): 3382, 2927, 1583, 1462, 1384, 1298, 1269, 1190, 1157, 1093, 1018, 745. H NMR Spectra [200 M Hz, CD3OD] delta (ppm): 8.23 - 8.25 (IH, d, ArH); 7.57 - 7.62 (2H, m, ArH); 7.0 - 7.09 (2H, m, ArH); 6.87 - 6.90 (IH, d, ArH); 4.57 - 4.63 (2H, d, O=S-CH2-Ar); 4.0 - 4.1 (2H, t, -O-CH2-CH2-); 3.49 - 3.55 (2H, t, -CH2-O-CH3); 3.31 (3H, s, -OCH3); 2.1 (3H, s, Ar-CH3); 1.96 - 2.0 (2H, t, -CH2-CH2-CH2-).; Example 2:Isolation of Rabeprazole sodium using dichloromethane as solvent:Rabeprazole (50 gm) was dissolved in solution of sodium hydroxide (5.4 gm) in demineralized water (150 ml). The solution was extracted with dichloromethane (100 ml) twice. The aqueous layer was then treated with neutral activated charcoal (1 gm) for 30 minutes at 28 to 35C. Carbon was removed by filtration and the residue washed with EPO <DP n="7"/>demineralized water (12.5 ml). Ethyl alcohol (50 ml) was added to the clear filtrate. The solution was concentrated to thick mass under vacuum at 40 - 450C. The thick mass was dissolved in ethyl alcohol (100 ml) and was again concentrated to a thick mass under vacuum at 40 - 45C. The residue was then dissolved in dichloromethane (150 ml) and the solution was filtered through 0.5-micron filter pad. The clear solution thus obtained was added slowly over a period of 20 to 30 minutes to diisopropyl ether (500 ml). The slurry thus obtained was stirred for 30 minutes at 28 to 35. The solid was filtered and washed with diisopropyl ether. Then the solid was dried at 45 - 50 to get Rabeprazole sodium as a white amorphous solid (as evident from powder X-ray diffraction of the product), with mean particle diameter ranging between 15 to 55mum. Yield: 44 gm (83 %), Assay: 99.5 % (by HPLC) |
| 72% |
With sodium hydroxide; In hexane; dichloromethane; at 10 - 20℃; for 4h; |
A solution of 10 - ((0.029mol) 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridyl]-methyl]thio]-1H-benzimidazole (condensate) Add 100ml toluene, 0.2 g of purified water, 8. lg (+) - L-diethyl tartrate, 4.5 g of titanium tetraisopropoxide, 52-55 C for lh; cooled to 25 C, 3.4 g of N, N-diisopropylethylamine, 4.5 g hydrogen peroxide cumene, 25-30 C reaction 2 h. After the oxidation reaction, with 2.5% sodium hydroxide solution extraction 2 times, each 60ml, combined with sodium hydroxide solution, Add lg algae and lg activated carbon decolorization, the filtrate with saturated ammonium chloride solution to rhoEta = 9.7, the control temperature of 10-20 C, precipitation of white solid, crystallization lh after filtration. A 16 g dextroride dexeprazole was obtained (dry 8 g (0.022 mol 1)). 120 ml of dichloromethane and 300 ml of n-hexane were mixed and 0.88 g (0.022 mol) of sodium hydroxide solid was added with stirring. The control temperature is 10-20 C, Add dextrorolidazole wet to the above mixture, add finished, continue to control temperature 10-20 C 4h. The reaction was completed, filtered, washed, vacuum dried at 60 C, and dexamethasone was dissolved in sodium 7.2 g, yield: 72% Purity: 99.81%, sulfone:0.01%. |
| 64.94% |
With sodium hydroxide; In methanol;Cooling with ice; |
39.76 g of rabeprazole was dissolved in 80 ml of methanol and added dropwise with sodium hydroxide in 80 ml of methanol under ice-cooling. After the addition was completed, the mixture was stirred at room temperature for 2 hours.Dried methanol, weighed 41.59 grams.Recrystallization from methyl tert-butyl ether and n-butanol, crystallization at room temperature, ice bath, filtration.Sampling measured content, sulfoxide content of 99.89%, sulfone content of 0.07%.The mixture was dried in vacuo and weighed 27.42 g in 64.94% yield.Rabeprazole sodium salt NMR spectrum shown in Figure 1, thermogravimetric analysis showed that the product contains 1.0 crystal water (Figure 2). |
|
With sodium hydroxide; In methanol; water; |
Example 2; Preparation of 2-[{4-(3-methoxypropoxy)-3-methylpyridine-2-yl}methylsulfinyl]-1H-benzimidazole Sodium Salt (Rabeprazole Sodium Salt); The product of example 1 is dissolved in 53.0 ml of 0.5 N and 0.1 % aqueous solution of methanolic sodium hydroxide. Obtained solution is concentrated and the residue is added to diethyl ether dropwise to precipitate 2-[{4-(3-methoxypropoxy)-3-methylpyridine-2-yl}methylsulfinyl]-1H-benzimidazole sodium salt. Percentage of product purity is 99.83 % and sulfone impurity in the bulk product is 0.07 %. |
|
With sodium hydroxide; In methanol; at 25℃; for 2h;Industry scale; |
Sodium hydroxide flakes (6.0 kg) and methanol (80 liters) were taken into a reactor and stirred for about 1 hour. The mixture was checked for clear dissolution and then rabeprazole (40kg) was added to the solution and stirred at about 25 0C for about 2 hours. The solution was checked for clear dissolution and distilled off completely under a vacuum of about 550 mm/Hg at a temperature of about 50 0C. The residue obtained was cooled to about 25 0C and n-butanol (20 liters) was added and stirred for about 25 minutes. Methyl tertiary butyl ether (160 liters) was then added and stirred at about 25 0C for about 10 minutes. Another 240 liters of methyl tertiary butyl ether was added to it and stirred for about 10 minutes. Rabeprazole sodium pure sample (0.8 kg) was added as a seed to the reaction mass and maintained at about 25 0C for about 12 hours. The reaction mass was then further <n="25"/>cooled to about 5 0C and maintained for about 3 hours. The separated solid was filtered and washed with methyl tertiary butyl ether (80 liters) under nitrogen atmosphere. The wet solid was dried at about 25 0C to about 30 0C for about 3 hours and then sieved and then again dried at about 50 0C to about 60 0C for about 4 hours. Finally, the solid was dried at a temperature of about 90 0C to about 95 0C for about 4 hours to about 5 hours. The material was further sifted in a No. 10 mesh, and finally pulverized to yield 36 kg of the title compound. Purity by HPLC: 99.83%. % of sulfone impurity: 0.07%. Particle Size analysis: D90: Less than 70 mum. Water by KF: 5.4% w/w. |
|
With sodium hydroxide; In water; 4-methyl-2-pentanone; at 20℃; |
25g of crude rabeprazole is added to 125ml of methyl isobutyl ketone and stirred to obtain a homogenous suspension. Aqueous sodium hydroxide is then added to this suspension with stirring over a period of about 10 minutes. The reaction mixture is stirred at room temperature for another 15-30 minutes until a biphasic reaction mixture is obtained. This reaction mixture is then distilled at 45-500C under vacuum to remove the solvents. The syrupy mass thus obtained is mixed with 50ml of methyl isobutyl ketone, distilled under vacuum at 45-500C and degassed for 30 minutes to obtain a residual mass. This residual mass is dissolved in 50ml of methyl isobutyl ketone to obtain a clear solution. This clear solution is added dropwise, with stirring, to a mixture containing 375ml of methyl t-butyl ether and 12.5ml of methyl isobutyl ketone. The mixture is stirred for about 1 to 2 hours at room temperature, followed by cooling to 0-50C for about 1 to 2 hours. The product obtained is filtered under nitrogen atmosphere and dried under vacuum at 45- 500C for about 10 hours. The amorphous rabeprazole sodium thus obtained contains 0.05% of the corresponding sulfone impurity, when analyzed by HPLC. |
|
With sodium hydroxide; In methanol; at 20℃;Product distribution / selectivity; |
Example 1: Rabeprazole sodium form A5.0 g (13.9 mmoles) of rabeprazole was dissolved in a solution of 0.75 g (18.7 mmoles) sodium hydroxide in 10 ml methanol at room temperature. Any undissolved particles were filtered off to get a clear solution. The solution was concentrated to an oily residue, 10 ml of methylene chloride was added and evaporated to dryness. The obtained dry product was dissolved in 30 ml of ethyl acetate, concentrated to 20 ml of volume and stirred for 12 hours at 20-250C. The suspension was cooled to 0-50C for one hour, filtered, washed with ethyl acetate. The product was dried in vacuo at 400C to obtain 5.1 g of crystalline form A. |
|
With sodium hydroxide; In methanol; at 25 - 35℃; for 1h;Industry scale; |
Sodium hydroxide (8.2 Kg) was dissolved in methanol (360 L). Rabeprazole was added to the solution and stirred at 25 - 350C for 1 hour. Methanol was distilled off from the reaction mass, dichloromethane (150 L) was added to the residual mass and the contents were stirred to obtain solution. The solution was added to cyclohexane (1080 L). The contents were stirred at 25 - 350C for 30 minutes, centrifuged the material and washed at 60 - 650C to obtain 69 Kg of amorphous rabeprazole sodium. |
|
With sodium hydroxide; In water; ethyl acetate; at 20℃; for 24h; |
EXAMPLE 3 2-[3-Methyl-4-(3-methoxypropoxy)-2-pyridinyl)methyl]sulfinyl}-1H-benzimidazole (I) (rabeprazole sodium salt) A 3-necked round-bottom flask equipped with reflux condenser, thermometer and magnetic stirring, under nitrogen atmosphere, is loaded with 2-[4-(3-methoxypropoxy)-3-methyl-1-oxypyridin-2-yl-methylsulfinyl]-1H-benzimidazole (intermediate II) (45.0 g; 111 mmols), methanol (270 ml), 30% sodium methoxide in methanol (20.1 g), tetramethylethylenediamine (77 g; 666 mmols) and RuCl3 hydrate (40% w/w Ru; 1.2 g; 5.5 mmols). The reaction mixture is heated to 50C for 10 hours, then analyzed by HPLC (95% yield in solution). The mixture is cooled to room temperature, concentrated to half volume and diluted with 500 ml of water. The formed precipitate is filtered through Celite. pH is adjusted to 6-7 first with acetic acid, then with sodium sulfite, and the mixture is extracted in ethyl acetate (300 ml). A sodium hydroxide 50% solution in water (440 mg; 111 mmols) is added to the mixture which is stirred for 24 hours at room temperature. The title product crystallized as polymorphic form beta, disclosed in, in a purity higher than 99.7% (yield 91 %). |
|
With sodium hydroxide; In methanol; at 20 - 30℃; for 2h; |
Charge 300 ml methanol and 11.12 g of sodium hydroxide in the reactor. Cool the reaction mixture to 20 to 3O0C. Charge 100 g Rabeprazole sulphoxide in the reaction and stir it at 20-300C for about 1 hour. Add 10 g charcoal to the reaction mixture and stir it for one hour at 20-300C. Filter the reaction mixture through hyflo bed. Distill out methanol under vacuum at 550C to obtain residue. Charge 100 ml toluene and distill out under vacuum at 550C. Charge 100 ml toluene and 50 ml ethyl acetate to obtain a clear solution. Add the solution to 1000 ml methyl tert. butyl ether. Stir the reaction mass for one hour to obtain amorphous form of Rabeprazole sodium. Filter the product and dry the product under vacuum at 55-6O0C (100 g). |
|
With sodium hydroxide; In water; isopropyl alcohol; at 15 - 20℃;Industry scale; |
24 kg of a 30% NaOH aqueous solution, 320 L of isopropyl alcohol and approximately 66 kg of rabeprazole were loaded in a reactor. The reaction mixture was kept at the temperature between 15 and 20C and the resulting solution was subsequently filtered. A vacuum of less than 100 mbar was applied and it was distilled at a temperature of less than 40C until obtaining a thick mass .50 L of isopropyl alcohol were then loaded and it was distilled again maintaining the same vacuum and temperature conditions. 50 L of acetone were loaded and the distillation process was repeated.150 L of acetone were then loaded and the resulting reaction mass was cooled to a temperature between 0 and 5C. The solid was filtered to finally obtain 56 kg corresponding to the acetone solvate of rabeprazole sodium salt.The product thus obtained has a DSC corresponding to Figure 1. The XRPD pattern of an acetone solvate of rabeprazole sodium salt is shown in Figure 2. |
|
With sodium hydroxide; In ethanol; at 20 - 25℃; |
Example 5 : Preparation of rabeprazole sodium Ethanol (325 mL) was added to sodium hydroxide (7.24 g) and the mixture was heated to 40C to 45C. The mixture was stirred at 40C to 45C and cooled to 20C to 25C. Rabeprazole (65 g) obtained in Example 4 was added to the reaction mixture and it was stirred at 20C to 25C. Activated carbon (3.25 g) was added to the reaction mixture and the mixture was stirred at 20C to 25C for 30 minutes. The mixture was filtered through celite and washed with ethanol (2 X 130 mL). The filtrates were combined and filtered. The filter paper was washed with ethanol (65 mL) and spray dried under following conditions. Inlet temperature : 110C to 120C Oxygen Level: Not more than 3.0% The solid obtained was dried at 60C to 65C at 10 mm to 20 mm Hg till the ethanol content is not more than 5000 ppm and water content was not more than 4.5% w/w to obtain the title compound. Yield: 0.95 w/w Content of Form III: 1.5 ppm HPLC purity: 99.7% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 25 - 30℃; for 0.5h;Product distribution / selectivity; |
Rabeprazole (10 g) was suspended in 30 ml THF and sodium hydroxide solution (1.11 g sodium hydroxide dissolved in 1 ml water) was added at 25-3O0C. Reaction mass was stirred for 30 minutes at 25-30C. Added activated charcoal (1.0 g) and stirred for 30 minutes at 25-3O0C. Filtered and washed charcoal bed with THF (20 ml). Distilled out THF under reduced pressure at 30-350C completely. Residue was dissolved in THF (20 ml). In a separate R B flask charged n- heptane (100 ml) at 25C. Added slowly above reaction mass in 15-20 minutes at 25-3O0C. Stirred at 25-3O0C for 2 hours. The product was filtered off under nitrogen atmosphere and wet cake was washed with n-heptane (2 X 20 ml). Dried under vacuum at 5O0C for 20-24 hours to obtain amorphous Rabeprazole sodium.Dry wt. = 9.2 gram HPLC purity: 99.54% ; Example-3:Preparation of amorphous Rabeprazole sodium:Rabeprazole (10 g) was suspended in 30 ml THF and added sodium hydroxide solution (1.11 g sodium hydroxide dissolved in 1 ml water) at 25-3O0C. Reaction mass was stirred for 30 minutes at 25-300C. Added activated charcoal (1.0 g) and stirred for 30 minutes at 25-300C. Filter and washed charcoal bed with THF (20 ml). Distilled out THF under reduced pressure at 30-350C completely. Dissolved residue in THF (20 ml). In a separate R B flask charged diisopropyl ether (100 ml) at 25C. Added slowly above reaction mass in 15-20 minutes at 25-3O0C. Stirred at 25-3O0C for 2 hours. The product was filtered off under nitrogen atmosphere and wet cake was washed with diisopropyl ether (2 X 20 ml). Dried under vacuum at 5O0C for 20-24 hours to obtain amorphous Rabeprazole sodium.Dry wt. = 9.2 gram HPLC purity: 99.41% |
|
With sodium hydroxide; In water; at 25 - 30℃; for 0.5h;Product distribution / selectivity; |
Rabeprazole (10 g) was suspended in 20 ml water and sodium hydroxide solution (1.11 g sodium hydroxide dissolved in 10 ml water) was added at 25-30C. Reaction mass was stirred for 30 minutes at 25-3O0C. Added activated charcoal (1.0 g) and stirred for 30 minutes at 25-300C. Filtered and washed charcoal bed with water (10 ml). Added 30 ml ethanol to filtrate and distilled out till get oily residue under reduced pressure at 30-350C. Again added 30 ml ethanol to the oily residue and distilled out completely under reduced pressure at 35C. To the residue added 100 ml diisopropyl ether and stirred at 25-3O0C for two hours. Filtered product under nitrogen atmosphere and washed with 20 ml diisopropyl ether. Dried under vacuum at 5O0C for 20-24 hours to obtain amorphous Rabeprazole sodium.Dry wt. - 9.4 gram HPLC purity: 99.80% |
| 16.2 g |
With sodium hydroxide; In methanol; at 20℃; for 1h; |
2.25 g of NaOH was dissolved in 100 mL of methanol, and 20 g of dextro-rapbeprazole was added thereto with stirring, and reacted at room temperature for 1 h. The reaction was completed, suction filtration, and the filtrate was taken, and concentrated to dryness under reduced pressure to give a mixture of the right-handed rabeprazole sodium. Add the resulting solid to 50 mL In acetone, stir at 40 to 50 C to dissolve, filter, and remove insoluble matter. The crystallization was stirred at about 10 C for 8 h. Filter by suction, wash with acetone, dry, The white solid was 16.2 g, the chemical purity was 99.98%, and the ee value was 99.99%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping