Alternatived Products of [ 1073560-68-5 ]
Product Details of [ 1073560-68-5 ]
| CAS No. : | 1073560-68-5 |
MDL No. : | MFCD32197258 |
| Formula : |
C47H52N4O7
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | GZMLKDJDSMXRGP-HCVICXAGSA-N |
| M.W : |
784.94
|
Pubchem ID : | 138911354 |
| Synonyms : |
|
Chemical Name : | (S)-2-((S)-1-((S)-2-((S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)(methyl)amino)propanamido)-2-cyclohexylacetyl)pyrrolidine-2-carboxamido)-3,3-diphenylpropanoic acid |
Safety of [ 1073560-68-5 ]
Application In Synthesis of [ 1073560-68-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 1073560-68-5 ]
- 1
-
 [ 124-09-4 ]
[ 124-09-4 ]

-
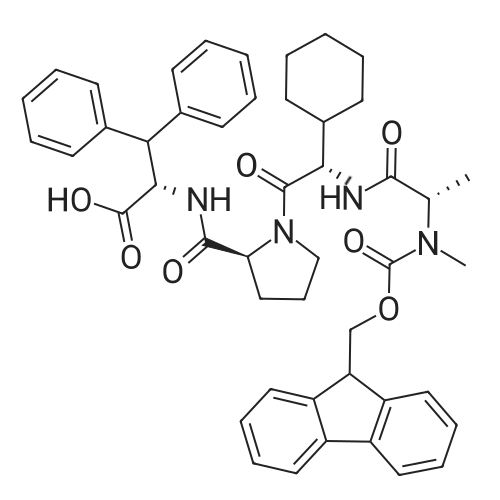 [ 1073560-68-5 ]
[ 1073560-68-5 ]

-
C100H116N10O12
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 20℃; for 14h; |
38
Example 38 dimer compound 8Compound a (160 mg, 0.2 mmol) was dissolved in 1 mL DMF and HATU (91 mg, 0.24 mmol) followed by addition of 1,6-diaminohexane (12 mg, 0.1 mmol) and diisoproplyethylamine (52 μL, 0.3 mmol). The reaction was stirred at room temperature for 14 hours. The reaction was diluted with EtOAc, washed 2x with saturated NaHCO3 and washed with brine. Dried over MgSO4 and concentrated. The residue was dissolved in 2 mL DMF followed by the addition of 4- aminomethylpiperidine (120 μL, 1.0 mmol) and stirred at room temperature for 3 hours. Preparative HPLC gave compound 8. MS = 1205.2 (M+l). |
- 2
-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]

-
 [ 109-76-2 ]
[ 109-76-2 ]

-
C97H110N10O12
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 20℃; for 14h; |
39
Example 39 dimer compound 9 Compound a (135 mg, 0.17 mmol) was dissolved in 1 mL DMF, HATU (91 mg, 0.24 mmol) was added followed by 1 ,6-diaminopropane (8 μL, 0.09 mmol) and diisoproplyethylamine (44 μL, 0.26 mmol). The reaction was stirred at room temperature for 14 hours. The reaction was diluted with EtOAc, washed 2x with saturated NaHCθ3 and washed with brine. Dried over MgSθ4 and concentrated. The residue was dissolved in 2 mL DMF followed by the addition of 4- aminomethylpiperidine (104 μL, 0.85 mmol) and stirred at room temperature for 3 hours. Preparative HPLC gave compound 8. MS = 1164.5 (M+l). |
- 3
-
C35H41N3O4
[ No CAS ]

-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 4 steps
1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 15 h / 20 °C
2: palladium on activated charcoal; hydrogen / 1,4-dioxane / 6.5 h / 20 °C
3: trifluoroacetic acid / dichloromethane / 3.5 h / 20 °C
4: potassium carbonate / tetrahydrofuran / 3 h / 20 °C |
|
Reference:
[1]Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]
- 4
-
 [ 1384275-58-4 ]
[ 1384275-58-4 ]

-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 3 steps
1: palladium on activated charcoal; hydrogen / 1,4-dioxane / 6.5 h / 20 °C
2: trifluoroacetic acid / dichloromethane / 3.5 h / 20 °C
3: potassium carbonate / tetrahydrofuran / 3 h / 20 °C |
|
Reference:
[1]Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]
- 5
-
C32H42N4O5
[ No CAS ]

-
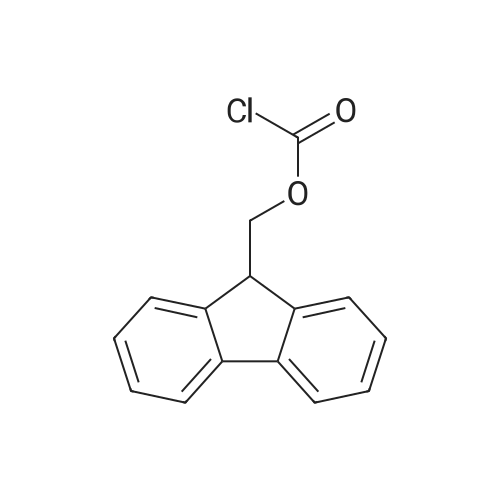 [ 28920-43-6 ]
[ 28920-43-6 ]

-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]
| Yield | Reaction Conditions | Operation in experiment |
| 56% |
With potassium carbonate In tetrahydrofuran at 20℃; for 3h; |
|
Reference:
[1]Location in patent: scheme or table
Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]
- 6
-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]

-
 [ 1384275-65-3 ]
[ 1384275-65-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 20 h / 20 °C
2: hydrogenchloride / 1,4-dioxane / 1 h / 20 °C |
|
Reference:
[1]Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]
- 7
-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]

-
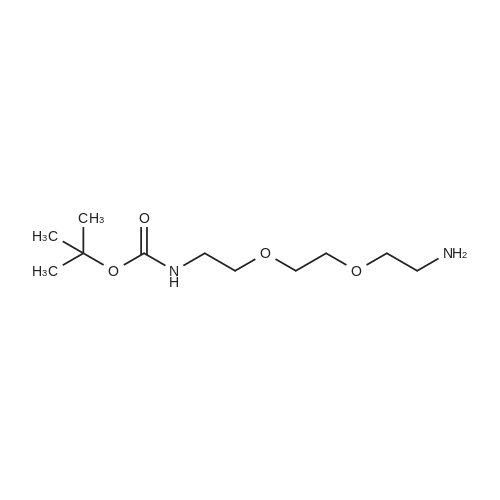 [ 153086-78-3 ]
[ 153086-78-3 ]

-
C58H74N6O10
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 20h; |
|
Reference:
[1]Location in patent: scheme or table
Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]
- 8
-
 [ 1384275-60-8 ]
[ 1384275-60-8 ]

-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 2 steps
1: trifluoroacetic acid / dichloromethane / 3.5 h / 20 °C
2: potassium carbonate / tetrahydrofuran / 3 h / 20 °C |
|
Reference:
[1]Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]
- 9
-
 [ 1384275-51-7 ]
[ 1384275-51-7 ]

-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 8 steps
1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 22 h / 20 °C
2: hydrogenchloride / 1,4-dioxane / 4.5 h / 20 °C
3: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 13 h / 20 °C
4: hydrogenchloride / 1,4-dioxane / 4.5 h / 20 °C
5: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 15 h / 20 °C
6: palladium on activated charcoal; hydrogen / 1,4-dioxane / 6.5 h / 20 °C
7: trifluoroacetic acid / dichloromethane / 3.5 h / 20 °C
8: potassium carbonate / tetrahydrofuran / 3 h / 20 °C |
|
Reference:
[1]Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]
- 10
-
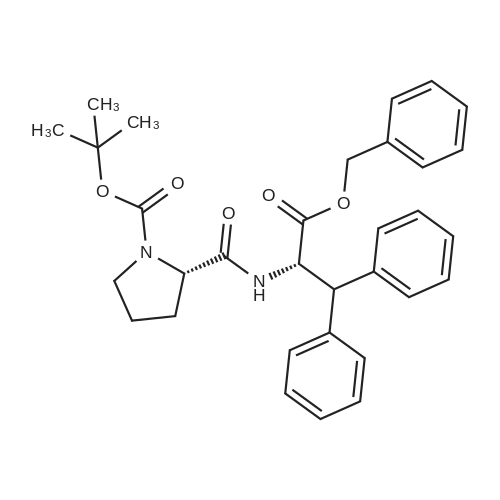 [ 1384275-54-0 ]
[ 1384275-54-0 ]

-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 7 steps
1: hydrogenchloride / 1,4-dioxane / 4.5 h / 20 °C
2: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 13 h / 20 °C
3: hydrogenchloride / 1,4-dioxane / 4.5 h / 20 °C
4: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 15 h / 20 °C
5: palladium on activated charcoal; hydrogen / 1,4-dioxane / 6.5 h / 20 °C
6: trifluoroacetic acid / dichloromethane / 3.5 h / 20 °C
7: potassium carbonate / tetrahydrofuran / 3 h / 20 °C |
|
Reference:
[1]Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]
- 11
-
C27H28N2O3
[ No CAS ]

-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 6 steps
1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 13 h / 20 °C
2: hydrogenchloride / 1,4-dioxane / 4.5 h / 20 °C
3: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 15 h / 20 °C
4: palladium on activated charcoal; hydrogen / 1,4-dioxane / 6.5 h / 20 °C
5: trifluoroacetic acid / dichloromethane / 3.5 h / 20 °C
6: potassium carbonate / tetrahydrofuran / 3 h / 20 °C |
|
Reference:
[1]Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]
- 12
-
 [ 1384275-56-2 ]
[ 1384275-56-2 ]

-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 5 steps
1: hydrogenchloride / 1,4-dioxane / 4.5 h / 20 °C
2: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 15 h / 20 °C
3: palladium on activated charcoal; hydrogen / 1,4-dioxane / 6.5 h / 20 °C
4: trifluoroacetic acid / dichloromethane / 3.5 h / 20 °C
5: potassium carbonate / tetrahydrofuran / 3 h / 20 °C |
|
Reference:
[1]Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]
- 13
-
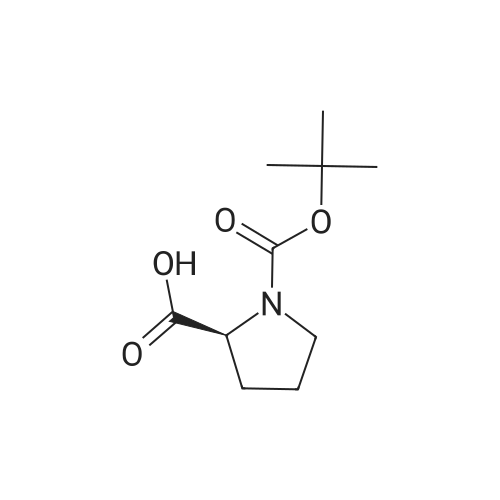 [ 15761-39-4 ]
[ 15761-39-4 ]

-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 8 steps
1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 22 h / 20 °C
2: hydrogenchloride / 1,4-dioxane / 4.5 h / 20 °C
3: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 13 h / 20 °C
4: hydrogenchloride / 1,4-dioxane / 4.5 h / 20 °C
5: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 15 h / 20 °C
6: palladium on activated charcoal; hydrogen / 1,4-dioxane / 6.5 h / 20 °C
7: trifluoroacetic acid / dichloromethane / 3.5 h / 20 °C
8: potassium carbonate / tetrahydrofuran / 3 h / 20 °C |
|
Reference:
[1]Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]
- 14
-
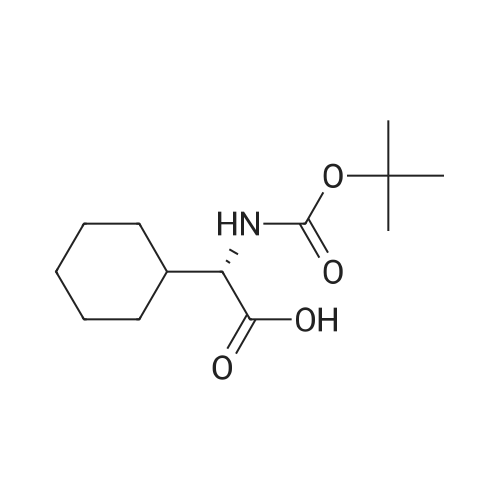 [ 109183-71-3 ]
[ 109183-71-3 ]

-
 [ 1073560-68-5 ]
[ 1073560-68-5 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Multi-step reaction with 6 steps
1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 13 h / 20 °C
2: hydrogenchloride / 1,4-dioxane / 4.5 h / 20 °C
3: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 15 h / 20 °C
4: palladium on activated charcoal; hydrogen / 1,4-dioxane / 6.5 h / 20 °C
5: trifluoroacetic acid / dichloromethane / 3.5 h / 20 °C
6: potassium carbonate / tetrahydrofuran / 3 h / 20 °C |
|
Reference:
[1]Itoh, Yukihiro; Ishikawa, Minoru; Kitaguchi, Risa; Okuhira, Keiichiro; Naito, Mikihiko; Hashimoto, Yuichi
[Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 13, p. 4453 - 4457]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping











