| 2.95 g |
With hydrogenchloride; water In tetrahydrofuran for 3h; Reflux; |
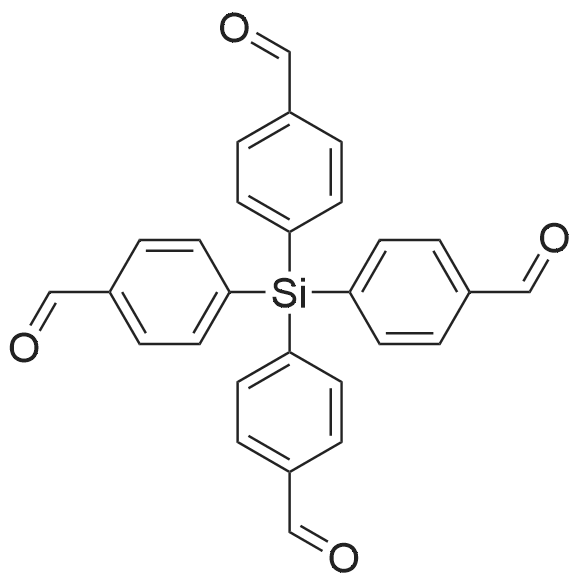
Tetrakis-4-formylphenylsilane (B).
To an oven-dried 500 mL round-bottom flask sealed with septum and magnetic stirbar under Ar atmosphere were added 150 mL of anhydrous THF using a cannula, and the flask was cooled to -80 oC with a liq. N2/EtOH bath. Next, 50 mL of tert-butyllithium (1.7 M in pentane, 85mmol, 2.2 eq) was added by cannula, followed by the dropwise addition of 4-bromobenzaldehyde dimethyl acetal (6.4 mL, 38.6 mmol, 1 eq). The reaction was stirred for 3 hrs at -80 oC before silicon tetrachloride (0.9 mL,, 7.9 mmol, 0.2 eq) was added dropwise to the stirred suspension. The reaction was held at -80 oC for 3 additional hours, then allowed to warm to room temperature overnight. The reaction was quenched with 100 mL of 1M HCl, and extracted 3 times with diethylether. The combined organic phases were washed once with 100 mL of deionized water, and once with saturated NaCl, and concentrated by rotary evaporation to give a dark reddish oil. The oil was dissolved in 60 mL of THF and the solution was added to a 250 mL round bottom flask containing a stirbar and 60 mL of 2M HCl. The mixture was rapidly stirred and heated to reflux for 3 hours. Once it was cooled to room temperature, the mixture was extracted three times with 100 mL ethyl acetate. The combined organic fractions were washed once with 50 mL of 1M HCl, twice with 100 mL of deionized water, once with sat. NaHCO3, and once with sat. NaCl. The solvent was removed by rotary evaporation to give a dark amber thick oil. This oil contained the small amount of excess benzaldehyde and arylsilanols and siloxanes by-products that greatly inhibited crystallization of the product. The product was purified by column chromatography (EtOAc/hexanes on silica) or by filtering a CH2Cl2 solution through a filter pad as previously described for A. The purified product was recrystallized from 3:1 hexanes:ethyl acetate by dissolving the crude solid in 25 mL of hot ethyl acetate and adding 75 mL of boiling hexanes in three portions, which caused tiny white crystals to form immediately. The solution was cooled to room temperature and then was placed in the freezer overnight before the crystals were collected by filtration and washed with cold hexanes to give 2.95 g of product as small white crystals (6.6 mmol, 83.5%). The crystalline product contained traces of water, as evidenced by NMR and FTIR. 1H NMR (400 MHz, CDCl3) δ 10.09 (s, 1H), 7.94 (d, J = 7.8 Hz, 2H), 7.72 (d, J = 7.8 Hz, 2H), 1.59 (s, H2O). 13C NMR (101 MHz, CDCl3) δ 192.0, 139.5, 137.7, 136.8, 129.1. Mp 200-202 oC (lit. 200-204 oC). i FT-IR: 1703 cm-1 (CHO). Anal. Calcd for C28H20O4Si0.25H2O (B0.25H2O): C, 74.23; H, 4.56. Found: C, 74.20; H 4.55. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping





