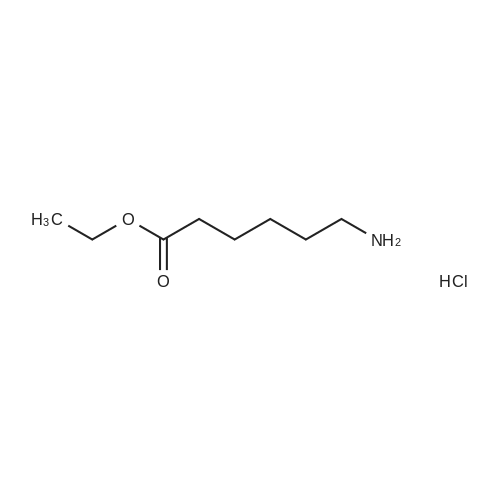
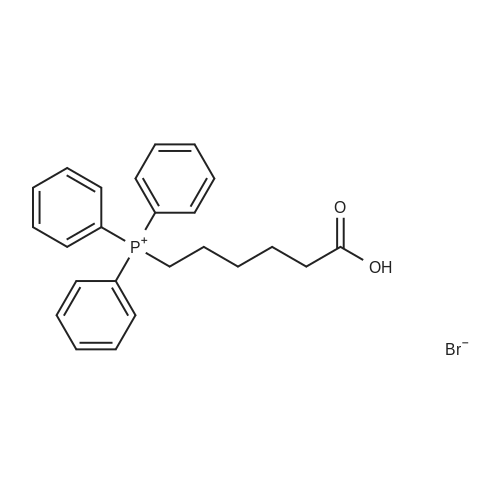
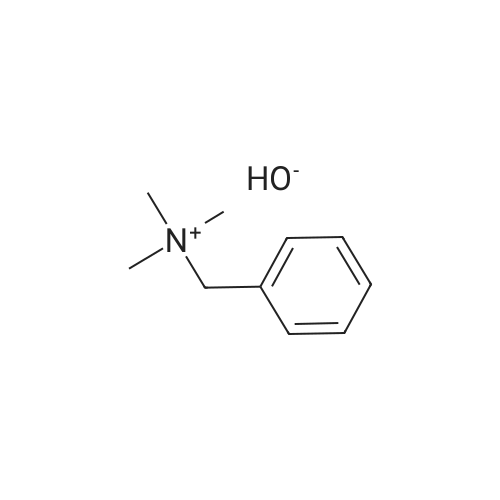
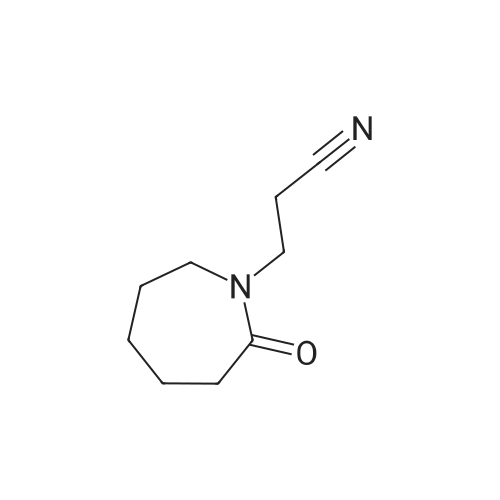
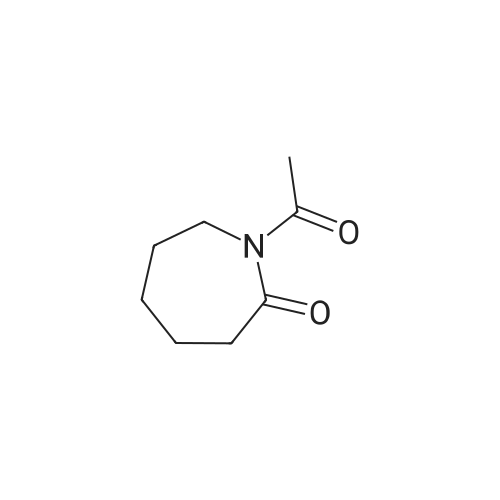
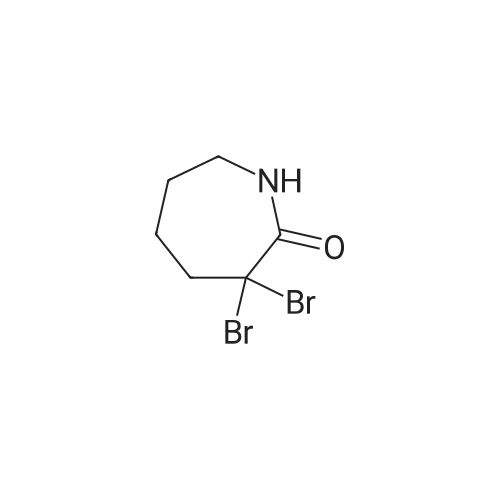
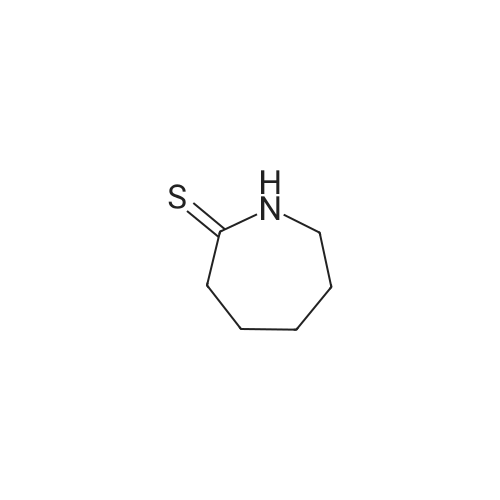
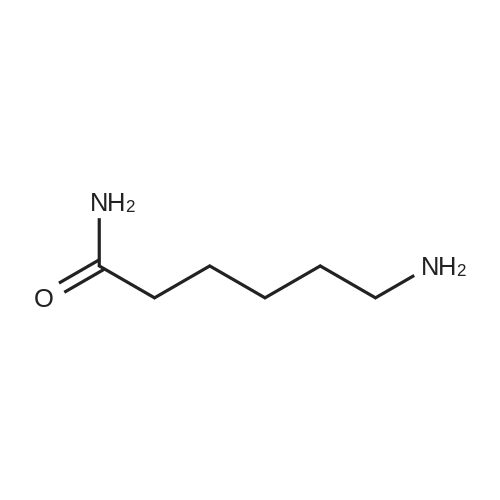
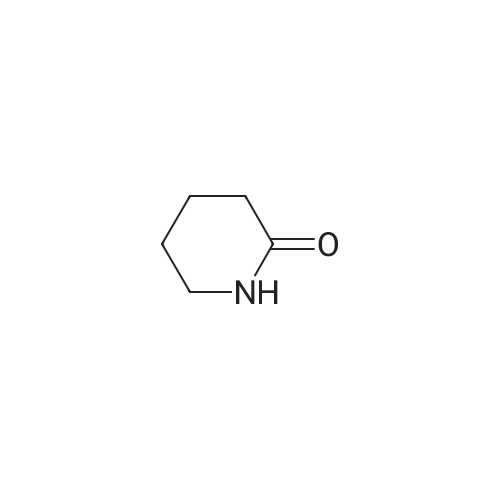
| 40% |
With hydrogen sulfide; hydrogen;Au-S/C (8 molpercent); at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
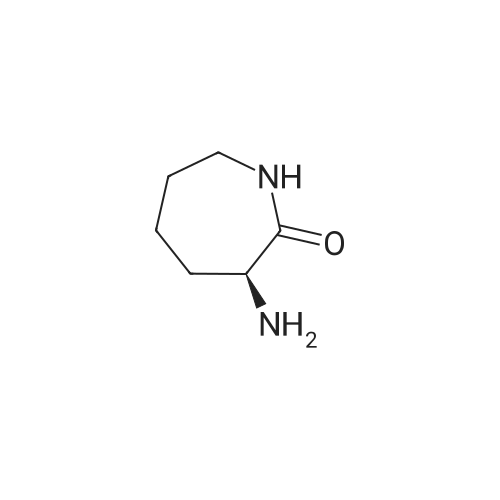
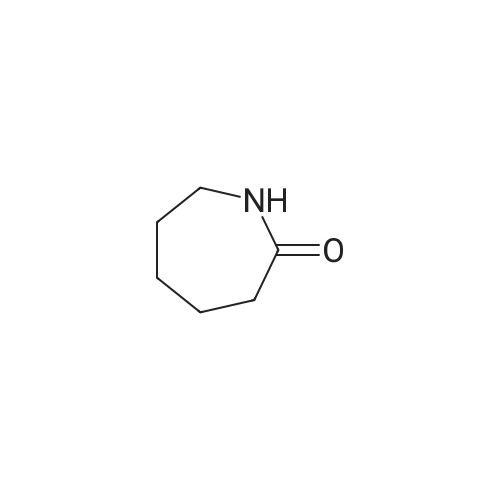
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 39% |
With hydrogen sulfide; hydrogen;Rh-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 37% |
With hydrogen sulfide; hydrogen;Pd-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 36% |
With hydrogen sulfide; hydrogen;8 % Pd/C; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 35% |
With hydrogen sulfide; hydrogen;8% Ru/C; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 35% |
With hydrogen sulfide; hydrogen;8% Rh/C; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 35% |
With hydrogen sulfide; hydrogen;Ni-Mo-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 34% |
With hydrogen;Pt-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 33% |
With hydrogen sulfide; hydrogen;Ir-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 26% |
With hydrogen sulfide; hydrogen;Re-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 25% |
With hydrogen;Rh-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 24% |
With hydrogen sulfide; hydrogen;8 % platinum on carbon; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 24% |
With hydrogen;Pd-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 23% |
With hydrogen;Ru-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 22% |
With hydrogen;8 % Pd/C; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 22% |
With hydrogen sulfide; hydrogen;Ni-Mo/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 20 - 65% |
With hydrogen sulfide; hydrogen;Pt-S/C (2-16 molpercent); In tetrahydrofuran; at 210 - 290℃; under 5171.62 - 7757.43 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 20% |
With hydrogen;8% Rh/C; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 20 - 62% |
With hydrogen sulfide; hydrogen;Au-S/NiO (8 molpercent); at 250 - 300℃; under 2585.81 - 5171.62 Torr; for 4 - 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 19% |
With hydrogen;8 % platinum on carbon; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 18% |
With hydrogen;Re-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 18% |
With hydrogen;Ir-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 16% |
With hydrogen sulfide; hydrogen;8% Re/C; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 15% |
With hydrogen sulfide; hydrogen;Pt-S/C (4 molpercent); In 1,2-dichloro-benzene; at 250℃; under 7757.43 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 15% |
With hydrogen sulfide; hydrogen;nickel(II) oxide; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 14% |
With hydrogen sulfide; hydrogen;Pt-S/C (4 molpercent); In hexan-1-ol; at 250℃; under 7757.43 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 14% |
With hydrogen;8% Ru/C; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 14% |
With hydrogen sulfide; hydrogen;Au/NiO; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 13% |
With hydrogen sulfide; hydrogen;8% Ir/C; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 13% |
With hydrogen;Ni-Mo-S/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 10% |
With hydrogen;8% Re/C; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 7% |
With hydrogen sulfide; hydrogen;Pt-S/C (4 molpercent); In cyclohexane; at 250℃; under 7757.43 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 6% |
With hydrogen;Ni-Mo/C (8 molpercent); In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 4% |
With hydrogen sulfide; hydrogen;Pt-S/C (4 molpercent); In 2,5-dimethyltetrahydrofuran; at 250℃; under 7757.43 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
| 3% |
With hydrogen;8% Ir/C; In tetrahydrofuran; at 250℃; under 5171.62 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |
|
With potassium hydroxide; hydroxylamine-O-sulfonic acid; at -20 - -5℃;Product distribution / selectivity; |
After completion of the cyclization reaction (1) as described above, the NaCl is removed. 20 mmols of alpha-amino-No.-caprolactam 2 is placed in a reaction chamber and the temperature of the chamber is lowered below the freezing point of water to about-20C. 800 mmols of KOH is added and then 400 mmols of hydroxylamine-O-sulphonic acid is added. The amine is washed away using a solvent that is 240 ml of water and 160 ml of methanol. The product, s-caprolactam 3, is then purified by sublimation and the yield is about 61 % based on L-lysine starting material. Example 12 [0033] After completion of the cyclization -reaction (1) as described above, the NaCl is removed. 20 mmols of a-amino-E-caprolactam 2 is placed in a reaction chamber and the temperature of the chamber is lowered below the freezing point of water to about-20C. 800 mmols of KOH is added and then 400 mmols of hydroxylamine-O-sulphonic acid is added. The amine is washed away using a solvent that is 20 ml of water and 80 ml of methanol. The product, No.-caprolactam 3, is then purified by sublimation and the yield is about 62% based on L-lysine starting material. Example 13 [0034] After completion of the cyclization reaction (1) as described above, the NaCl is removed. 20 mmols of a-amino-s-caprolactam is placed in a reaction chamber and the temperature of the chamber is lowered below the freezing point of water to about-20C. 800 mmols of KOH is added and then 400 mmols of hydroxylamine-O-sulphonic acid is added. The amine is washed away using a solvent that is 60 ml of water and 40 ml of methanol. The product, s-caprolactam 3, is then purified by sublimation and the yield is about 64% based on L-lysine starting material. Example 14 [0035] After completion of the cyclization reaction (1) as described above, the NaCl is removed. 20 mmols of a-amino-E-caprolactam 2 is placed in a reaction chamber and the temperature of the chamber is lowered below the freezing point of water to about-20C. 160 mmols of KOH is added and then 80 mmols of hydroxylamine-O-sulphonic acid is added. The amine is washed away using a solvent that is 60 ml of water and 40 ml of methanol. The product, No.- caprolactam 3, is then purified by sublimation and the yield is about 65% based on L-lysine starting material. Example 15 [0036] After completion of the cyclization reaction (1) as described above, the NaCl is removed. 20 mmols of alpha-amino-No.-caprolactam 2 is placed in a reaction chamber and the temperature of the chamber is lowered below the freezing point of water to about-20C. 160 mmols of KOH is added and then 80 mmols of hydroxylamine-O-sulphonic acid is added. The amine is washed away using a solvent that is 60 ml of water and 40 ml of ethanol. The product, No.- caprolactam 3, is then purified by sublimation and the yield is about 70% based on L-lysine starting material.; Example 16 [0037] After completion of the cyclization reaction (1) as described above, the NaCl is removed. 20 mmols of alpha-amino-No.-caprolactam 2 is placed in a reaction chamber and the temperature of the chamber is lowered below the freezing point of water to about-5C. 160 mmols of KOH is added and then 80 mmols of hydroxylamine-O-sulphonic acid is added. The amine is washed away using a solvent that is 100 ml of water. The product, s-caprolactam 3, is then purified by sublimation and the yield is about 75% based on L-lysine starting material. |
| 0% |
With hydrogen sulfide; hydrogen;Pt-S/C (4 molpercent); In acetonitrile; at 250℃; under 7757.43 Torr; for 8h;Product distribution / selectivity; |
Catalytic Hydrodenitrogenation of alpha-Amino-epsilon-caprolactam. Under Ar, alpha-amino-epsilon-caprolactam (1.28 g, 10 mmol), THF (100 mL) and 16.3 wt% Pt-S/C (0.8 mmol) are added to the reaction chamber of a Parr high-pressure reactor and the vessel is assembled. The reaction chamber is flushed for 10 min with Ar and then pressurized with H2/H2S (5: 1) to 100 psi. The reaction chamber outlet valve is then opened to the atmosphere. This process is repeated two additional times. After repressurizing the reaction vessel with H2ZH2S (5: 1) to 100 psi, the temperature of the stirred reaction vessel is increased to 250C, which results in a reaction pressure of 650 psi. The stirred reaction vessel is held at to 2500C for 8 h. Upon cooling to rt, the pressurized reaction vessel's H2ZH2S atmosphere is vented through a bleach solution in a fume hood. After filtration, the reaction solution is concentrated, and the residue dissolved in EtOAc. The EtOAc solution is extracted with water followed by stirring the aqueous layer with activated carbon. Filtration and concentration affords crude caprolactam. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping