|
|
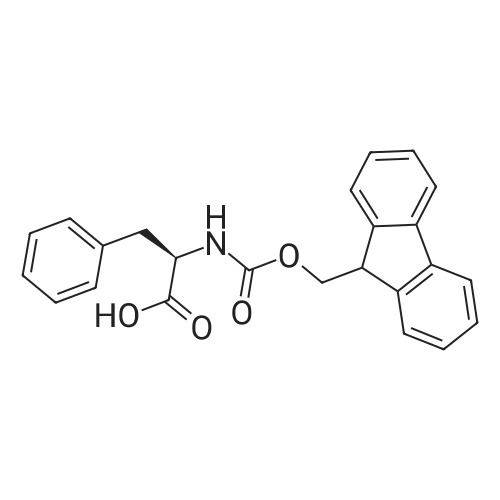
The starting resin Fmoc-L-Dab-L-Dab-L-Leu-2CT (prepared previously in a separate step, 418 mg, 0.198 mmol, 0.473 mmol/g) was pre-swollen in THF and then briefly washed with DCM (x 3) and DMF (x3). The resin was treated with a solution of 30% piperidine in DMF (ca. 1 0 mL per gram of resin) at ambient temperature (1 x 1 0 min, 1chi20 min). The resin was washed with anhydrous DCM (x 3) and then treated with Fmoc-aza- Leu-COCI, prepared as follows: Fmoc-aza-Leu (1 96 mg, 0.631 mmol) in anhydrous DCM (5 mL) was cooled to 0C and treated with a 20% solution of phosgene in toluene (665 mu, 1 .26 mmol, 2 equiv.). After 1 5 min, TLC indicated the reaction had gone to completion. The solution was concentrated and dried under vacuum to yield a colourless oil. The oil was redissolved in a solution of DCM (4 mL) and DIPEA (220 mu), and the resultant solution was added to the peptide resin. After shaking for 2 hr, the resin was drained and successively washed with DCM, DMF, MeOH and DCM (x 3 each), and dried under high vacuum overnight. The resin 2a yield was 420 mg (SV 0.458 mmol/g).General method for coupling Fmoc-amino acid to aza-amino acid: Resin 2a (420 mg, - 0.1 9 mmol) was treated with a solution of 30% piperidine in DMF (ca. 1 0 mL per gram of resin) at ambient temperature (1 x 1 0 min, 1chi20 min). The resin was washed with DMF followed by anhydrous THF (x 3 each). The resin was suspended in a minimal volume of THF and treated with DIPEA (300 mu). After 1 min, the resin was drained, re-suspended in THF (minimal volume), and treated successively with DIPEA (300 mu) followed by Fmoc-Phe-COCI, prepared as follows: Fmoc-Phe-OH (234 mg, 0.604 mmol, ~ 3 equiv. wrt resin) and BTC (56 mg, 0.1 88 mmol) in anhydrous THF (2 mL) was cooled to 0C and treated with 2,4,6-collidine (260 mu, 1 .98 mmol, 1 0 equiv. wrt resin). After 1 min, the solution was warmed to RT, stirred for 5 min and then added to the resin. After shaking for 3 hr, the resin was drained and successively washed with DMF and DCM (x 3 each), and dried under high vacuum overnight. The coupling was incomplete by lc/ms analysis. A second coupling was performed for an additional 4 hr. After washing and drying, the resin 2b yield was 420 mg (SV 0.429 mmol/g). The remainder of the peptide was synthesised using the standard peptide coupling procedure outlined for octapeptin C4. The final weight of the resin 2c was 408 mg (SV 0.33 mmol/g).Orthogonal N-deprotection: Resin 2c obtained above (408 mg) was pre- swollen in THF for 30 min. The solvent was drained, and the resin was agitated with a solution of 4% hydrazine hydrate in DMF (1 .3 mL, ca. 8 equiv.) for 1 h at ambient temperature. The solvent was drained, and the process repeated with an additional volume of 4% hydrazine hydrate in DMF (2 mL). The resin was successively washed with DMF (x 3), THF (x 3), IPA (x 3), DCM (x 3) and IPA (x 3). The resulting deprotected resin was dried under vacuum overnight. The final weight of the resin 2d was -400 mg (SV 0.36 mmol/g).Resin cleavage: The resin obtained above (400 mg, 0.36 mmol/g, 0.1 4 mmol) was treated with a solution of hexafluoroisopropanol (HFIP) in DCM (1 :4, 1 5 mL) for 2h. The solvent was drained, and the resin was washed with DCM (x 3). The filtrates were pooled, evaporated and then dried under vacuum overnight to afford crude 2d (123 mg) as a cream solid, in 6.8 (method 1 ). (ES) m/z 1463.8 (MH+). The material was used without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping