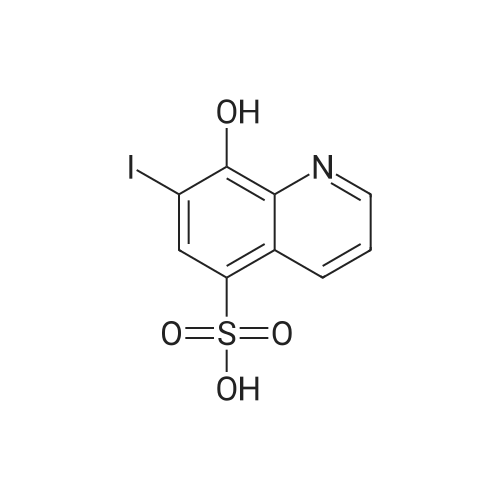
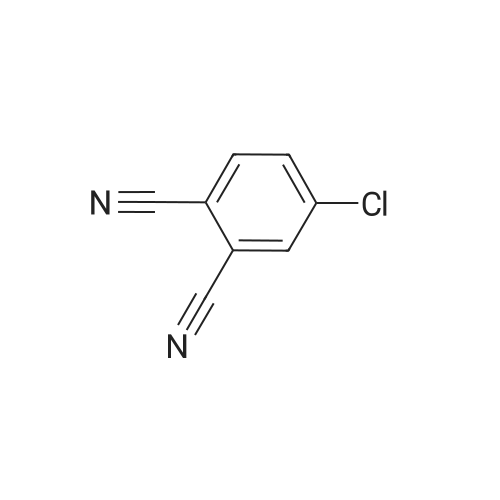
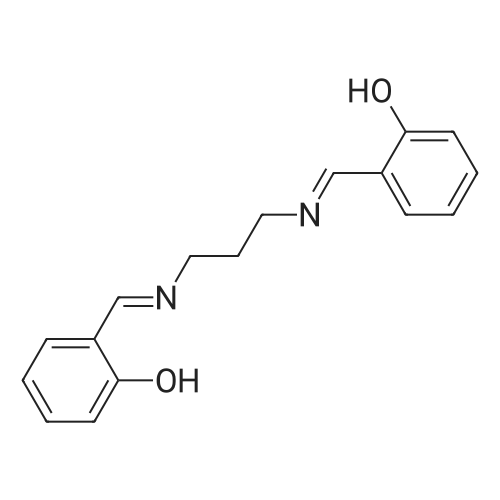
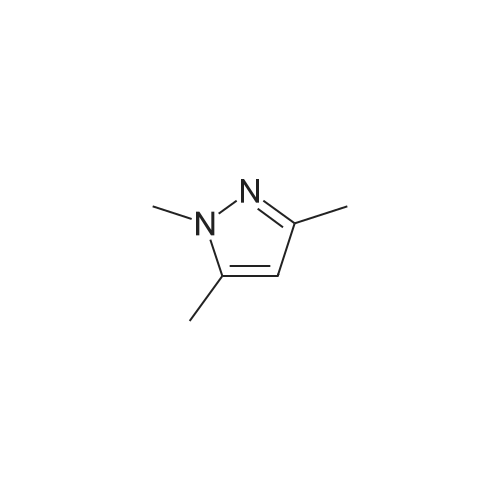
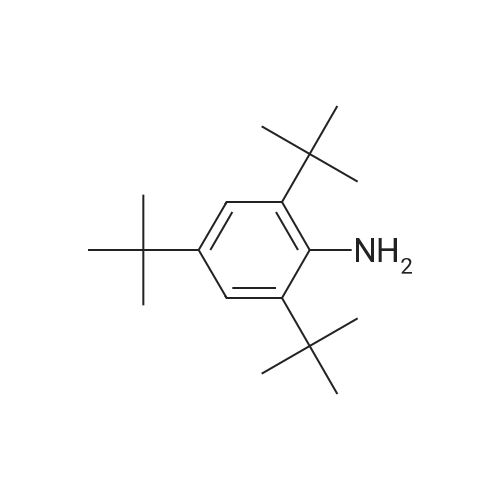
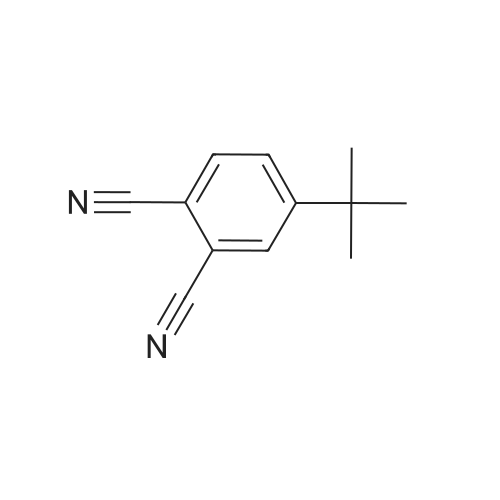
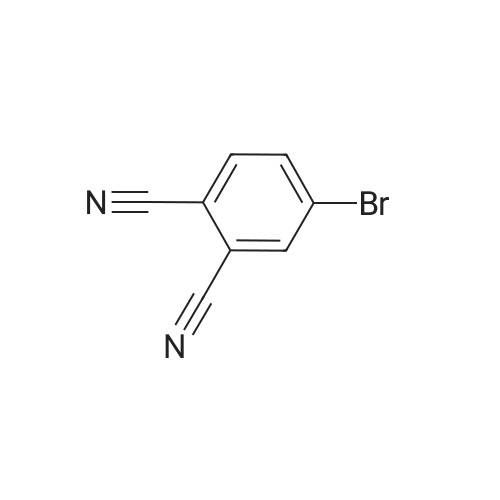
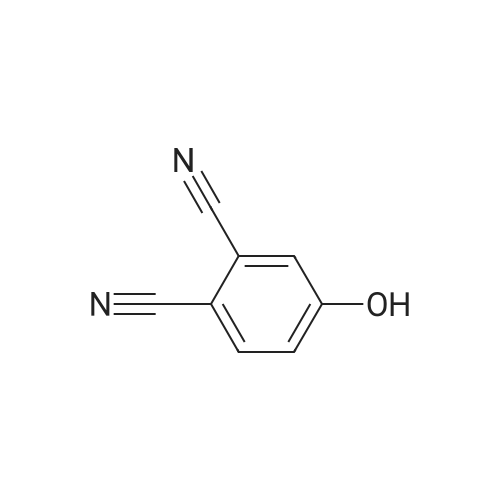
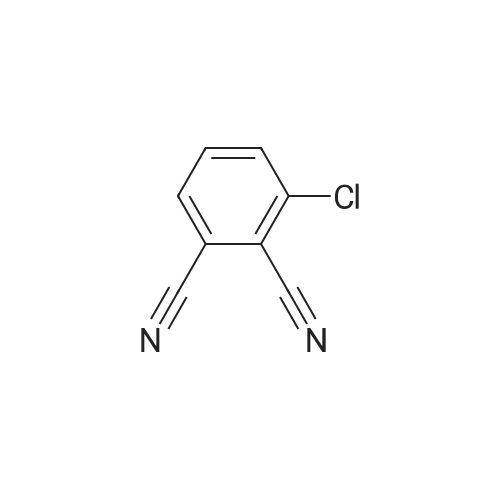
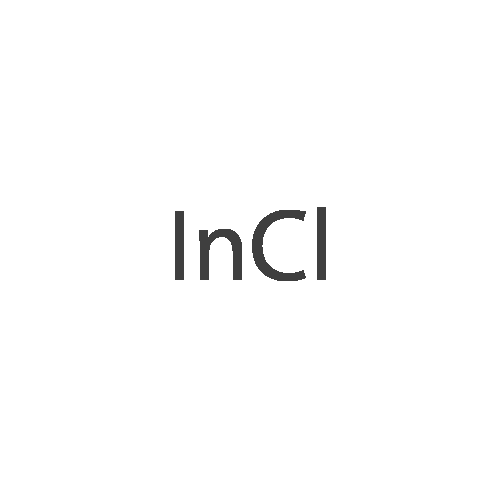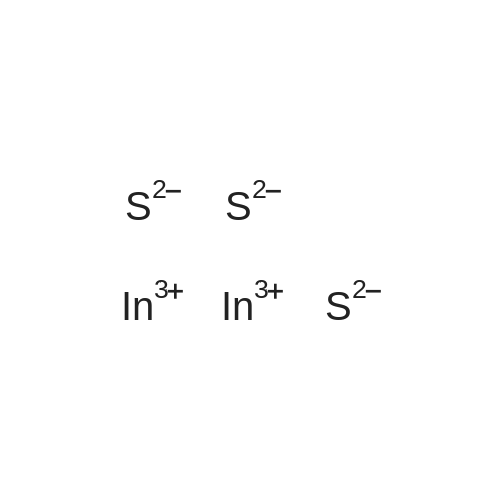|
With sodium cyanide; zinc In not given Electrochem. Process; electrolytical deposition of In on Zn from soln. of (in g/l): 32.8 In (as chloride), 90 NaCN, 15 dextrose; anode: carbon or Pt; current density: 1 - 11 A/dm*dm;; |
|
|
With zinc In ethanol High Pressure; anhydrous InCl3 and Zn powder added to a Teflon-lined stainless steel autoclave; autoclave filled with 90% absolute ethanol and the solution stirred for 30 min; autoclave sealed and maintained at 120°C for 20 h; cooled to room temp. naturally; product filtered, washed several times with distilled water and absolute ethanol; dried at 70°C for 4 h; |
|
|
In gaseous matrix metal compd. in discharge tube, passing an intense current pulse throught it (produced by discharging a capacitor across the tube, E=0.15-0.20 kJ), ballast gas N2; according to V. G. Mishakov et al., Opt. Spektrosk.32, 1006 (1972); density of metal atoms detd. by Rozhdestvenskii hook method; |
|
|
In not given Electrolysis; in the presence of formic acid; |
|
|
In not given Electrolysis; in the presence of hydroxylamine-HCl; |
|
|
In not given Electrolysis; in the presence of hydroxylamine; |
|
|
With poly(vinyl pyrrolidone); sodium tetrahydroborate In further solvent(s) InCl3 and poly(vinyl pyrrolidone) dissolved in isopropanol, purged with Ar, a soln. of NaBH4 in tetraethylene glycol added dropwise at room temp., stirred for 10 min; centrifuged, washed (EtOH); obtained as nanoparticles; |
|
|
With sodium naphthalenide; trioctylphosphine or trioctylamine In tetrahydrofuran byproducts: NaCl; room temp.; centrifugation, TEM, XRD; |
|
|
With potassium citrate In water Electrolysis; pH 2-5, current density 2-25 mA/cm**2, 30°C, metal ion concn. 0.2 mol/l; purity of the In is better than 99 %; |
|
|
With acetamide In water Electrolysis; pH 2-5, current density 2-25 mA/cm**2, 30°C, metal ion concn. 0.2 mol/l; purity of the In is better than 99 %; |
|
|
With acetate In water Electrolysis; pH 2-5, current density 2-25 mA/cm**2, 30°C, metal ion concn. 0.2 mol/l; purity of the In is better than 99 %; |
|
|
With chloride In water Electrolysis; pH 2-5, current density 2-25 mA/cm**2, 30°C, metal ion concn. 0.2 mol/l; purity of the In is better than 99 %; |
|
|
With ethanol In water Electrolysis; pH 2-5, current density 2-25 mA/cm**2, 30°C, metal ion concn. 0.2 mol/l; purity of the In is better than 99 %; |
|
|
With iodide In water Electrolysis; pH 2-5, current density 2-25 mA/cm**2, 30°C, metal ion concn. 0.2 mol/l; purity of the In is better than 99 %; |
|
|
With oxalate In water Electrolysis; pH 2-5, current density 2-25 mA/cm**2, 30°C, metal ion concn. 0.2 mol/l; purity of the In is better than 99 %; |
|
|
With sodium citrate In water Electrolysis; pH 2-5, current density 2-25 mA/cm**2, 30°C, metal ion concn. 0.2 mol/l; purity of the In is better than 99 %; |
|
|
With sulphate In water Electrolysis; pH 2-5, current density 2-25 mA/cm**2, 30°C, metal ion concn. 0.2 mol/l; purity of the In is better than 99 %; |
|
|
With thiocyanate In water Electrolysis; pH 2-5, current density 2-25 mA/cm**2, 30°C, metal ion concn. 0.2 mol/l; purity of the In is better than 99 %; |
|
|
With {(Li((CH3)2NC2H4N(CH3)2))2((CH3)3SiCHCH)2} In diethyl ether byproducts: LiCl, ((CH3)3SiCHCH)2; Ar or N2 atm.;; |
|
|
In water Electrochem. Process; electrodeposition (Bi electrode substrate, pH 1.3, current density 2.4-21.4 A/sqm, 1.2 - 8.3 h); |
|
|
In hydrogenchloride Electrochem. Process; electrodeposition (Sb electrode subtrate, pH 1.3, current densities between 2 - 20 A/sqm, room temp.); scanning electron microscopy; |
|
|
In further solvent(s) Electrolysis; electrolysis of 0.1 M InCl3 in 1-butyl-1-ethyl-piperidinium bis(trifluoromethylsulfonyl)imide at 60°C and potential from -100 to -1080 mVvs. AgCl/Ag; XRD; SEM; |
|
|
In further solvent(s) Electrochem. Process; deposited on Ni from 1-ethyl-3-methylimidazolium chloride-tetrafluoroborate ionic liquid at 120°C; |
|
|
In further solvent(s) Electrochem. Process; electrodeposited at 25°C at -1.75 V versus Pt from 1-butyl-1-methyl pyrrolidinium bis(trifluoromethylsulfonyl)amide containing InCl3; |
|
|
With LiCl In water Electrochem. Process; electrodeposited onto Mo/Cu at pH 3; |
|
|
With HCl; citric acid; sodium citrate In water Electrochem. Process; according to Phys. Chem. Chem. Phys. 13(2011)6662-6669, US Patent 20090283415 (2009); electrodeposited at 24+/-1°C from 30 mM InCl3 + 10 mM citric acid + 35 mM Na citrate (pH 2.2, HCl) at potential -0.98 V (vs. Ag/AgCl) (charge 0.96 C/cm2); |
|
|
With HCl; citric acid In water Electrochem. Process; according to Phys. Chem. Chem. Phys. 13(2011)6662-6669, US Patent 20090283415 (2009); electrodeposited potentiostatically at 24+/-1°C from 30 mM InCl3 + 10 mM citric acid (pH 2.2, HCl) at potential -0.98 V (vs. Ag/AgCl) (charge 0.96 C/cm2); |
|
|
With HCl In water Electrochem. Process; according to Phys. Chem. Chem. Phys. 13(2011)6662-6669, US Patent 20090283415 (2009); electrodeposited potentiostatically at 24+/-1°C from 30 mM InCl3 (pH 2.2 adjusted with HCl) at potential of -0.80 V (vs. Ag/AgCl) and charge of 0.96 C/cm2; |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping