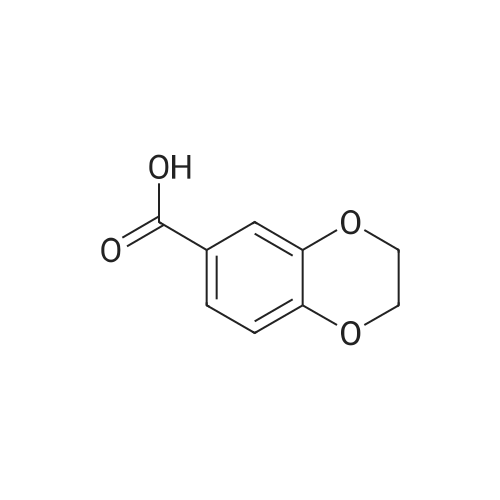
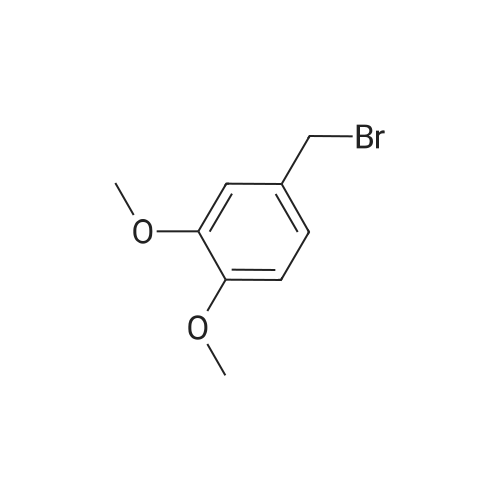
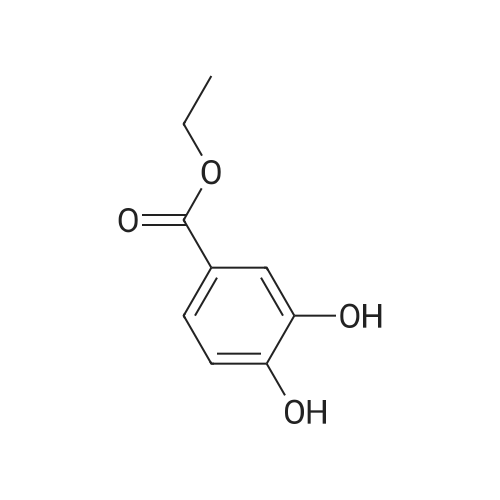
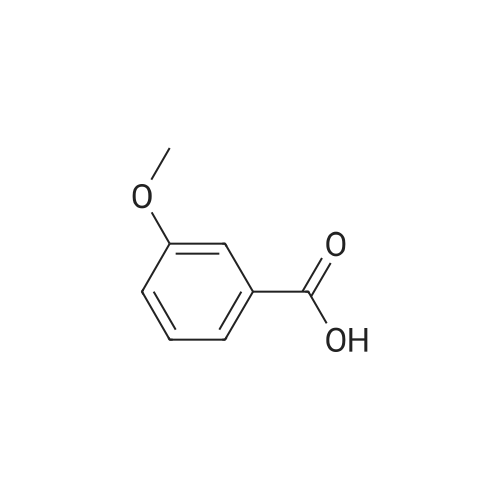
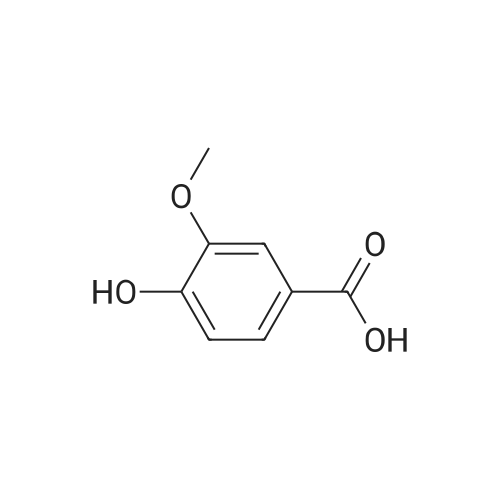
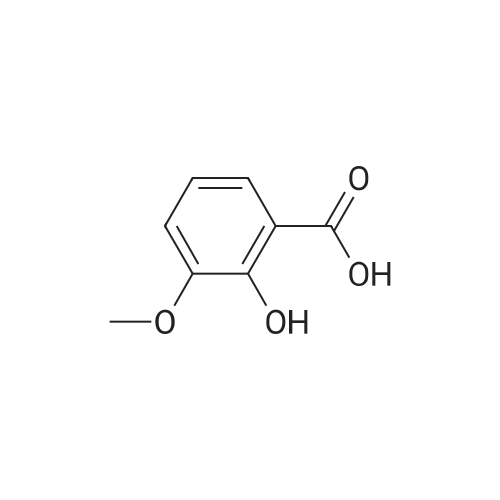
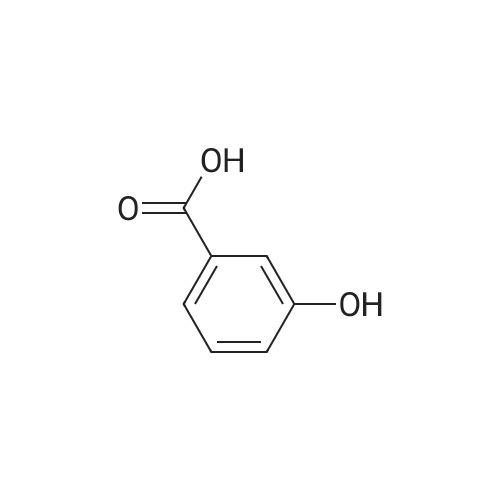
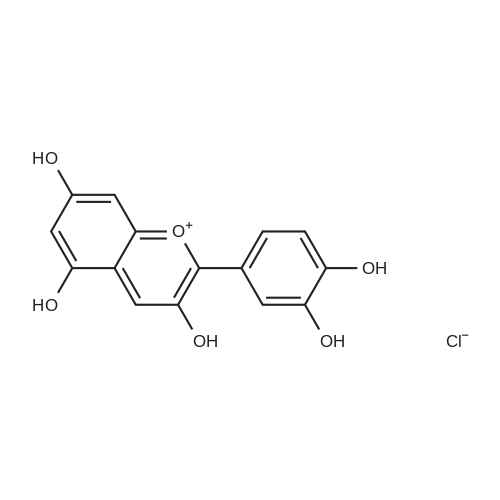
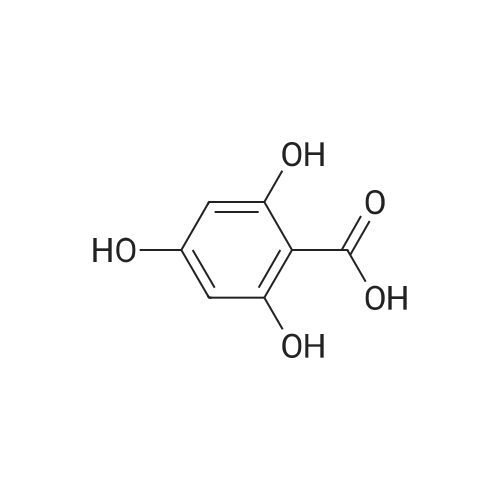
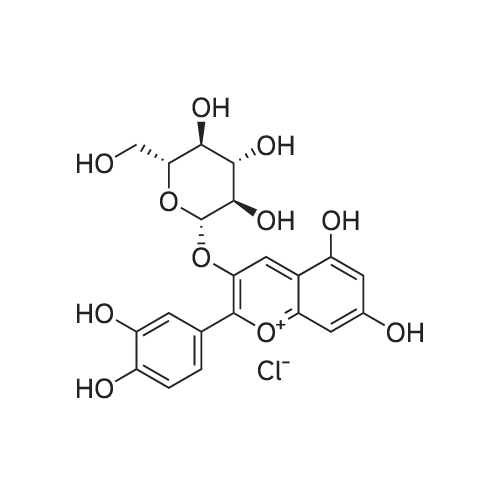
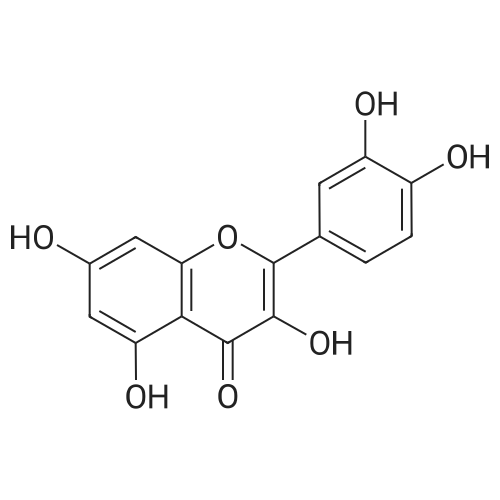
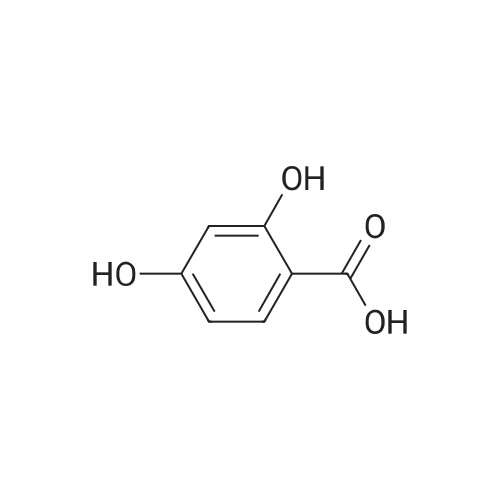
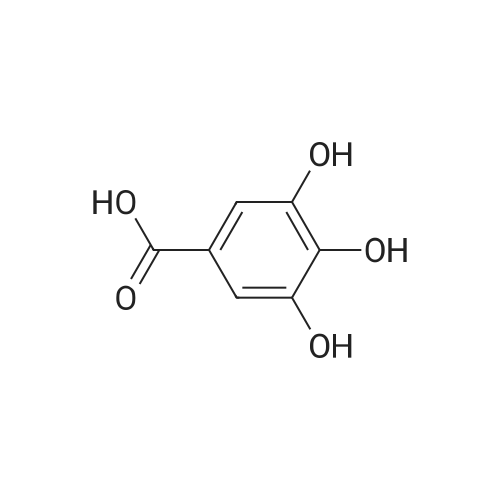
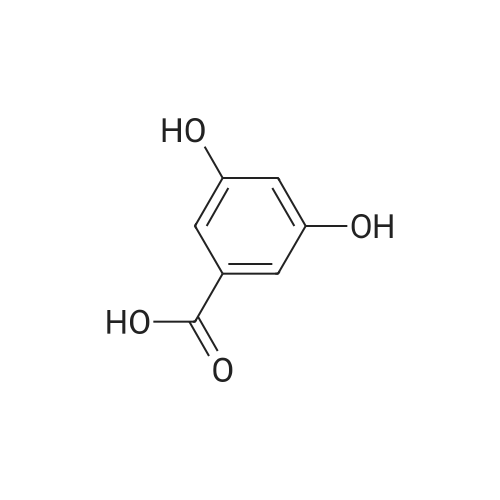
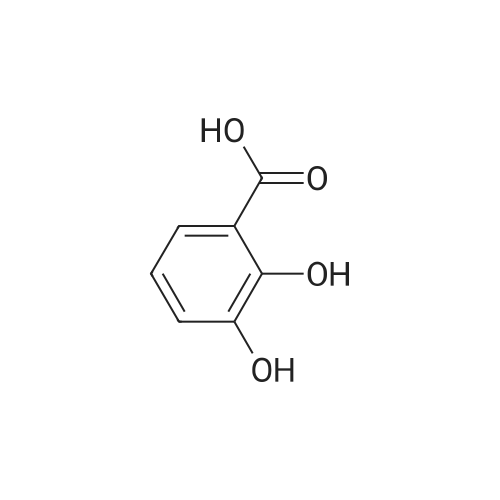
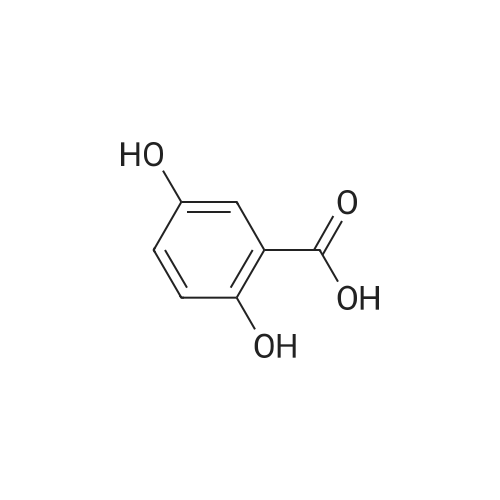
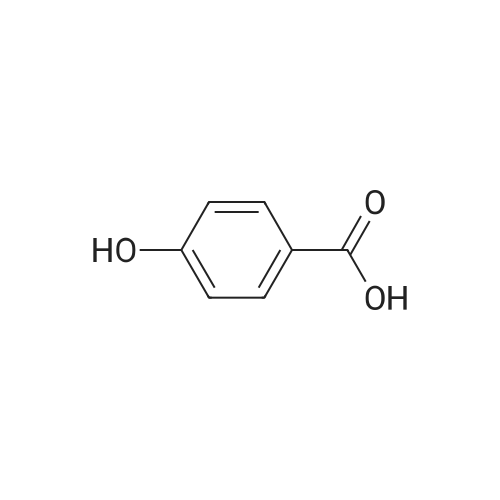
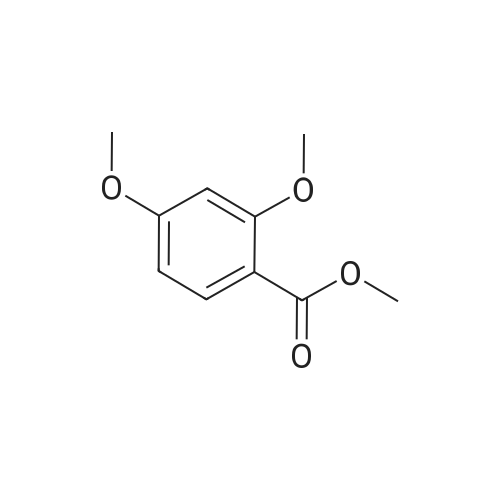
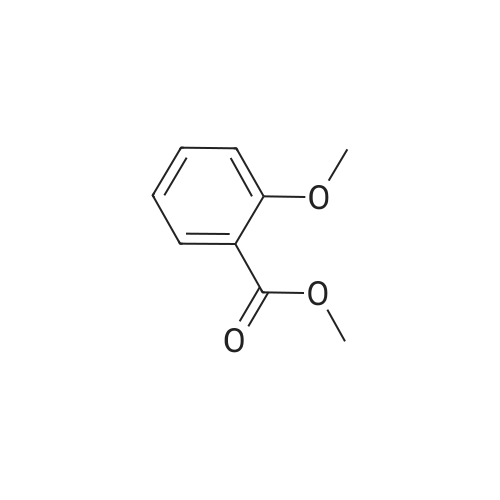
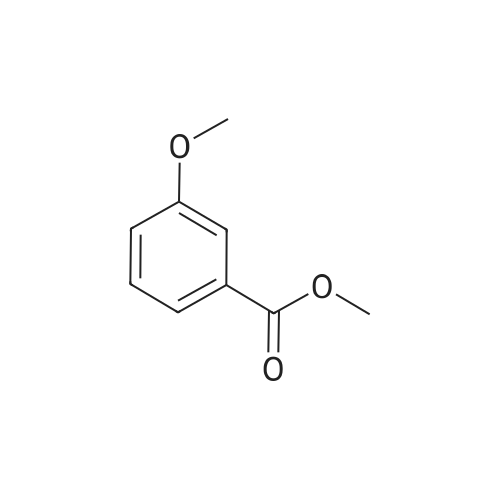
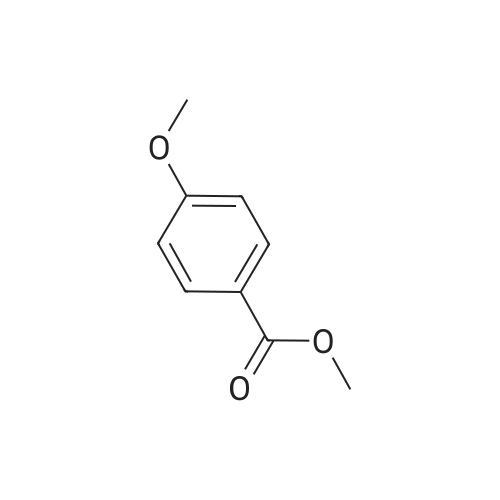
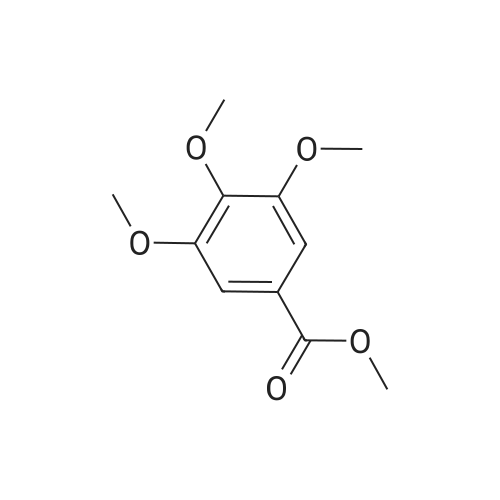
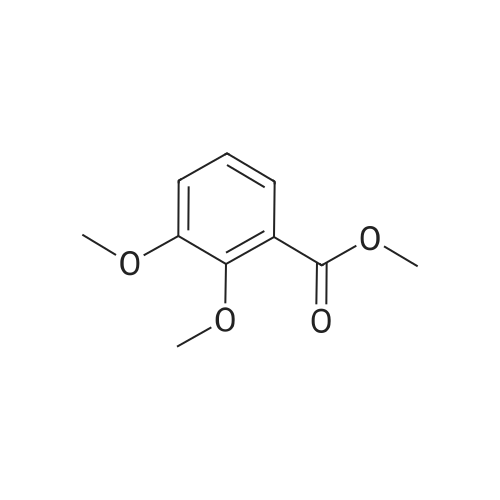
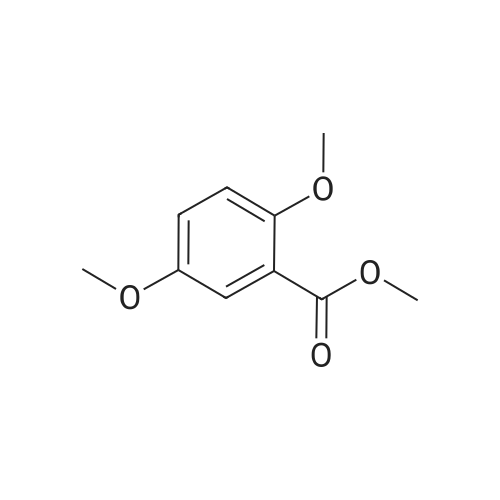
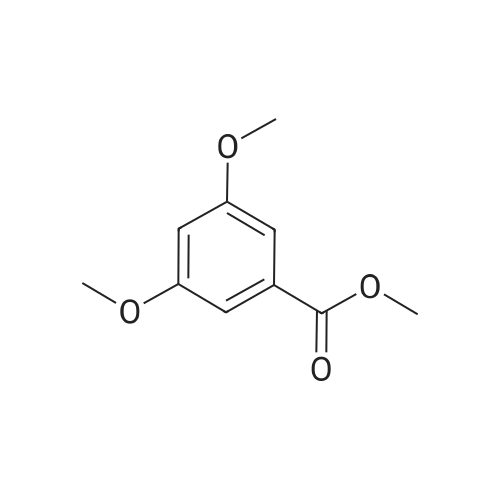
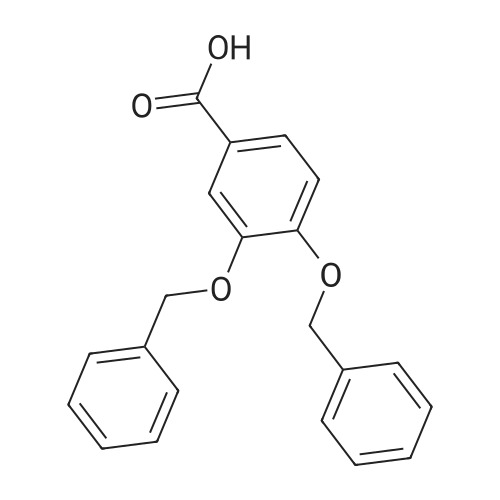
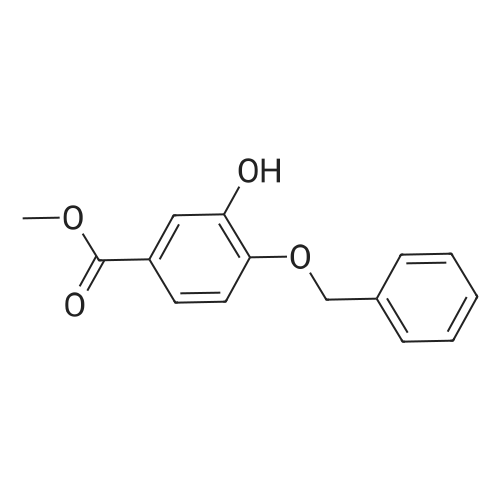
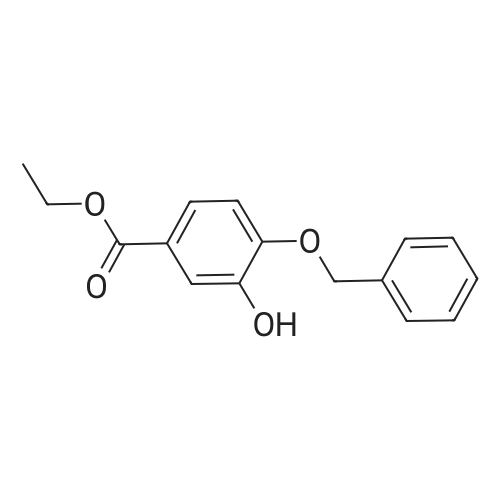
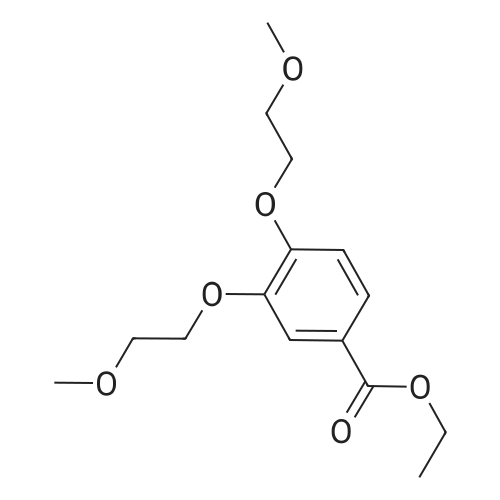
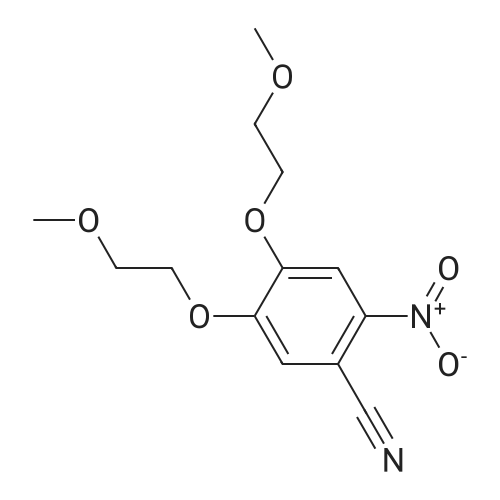
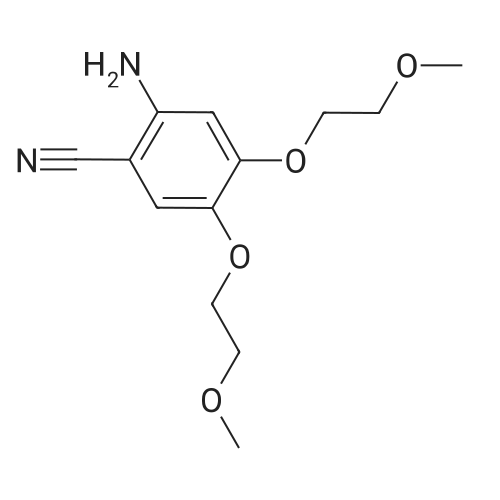
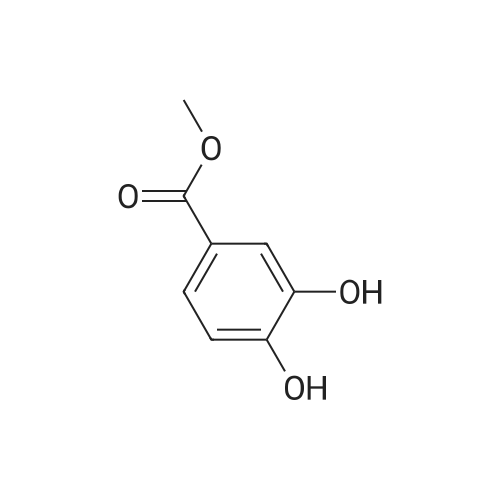
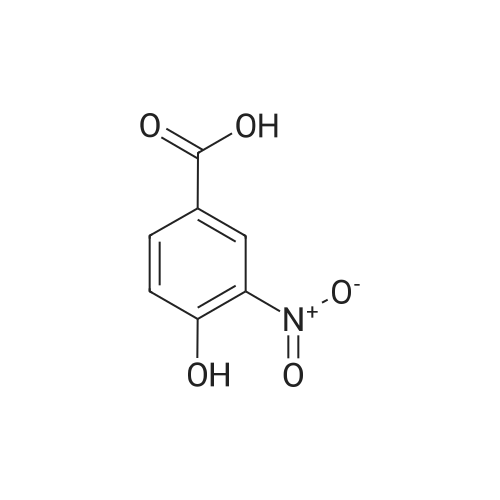
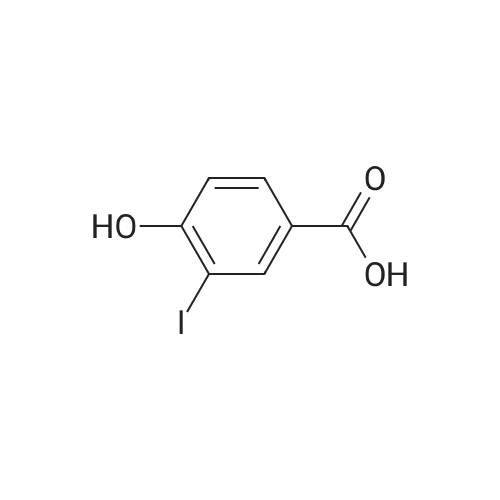
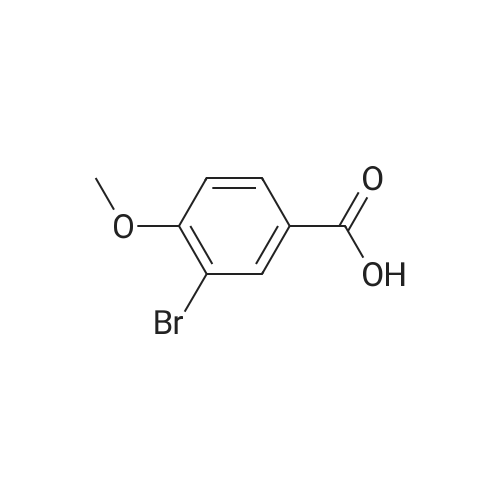
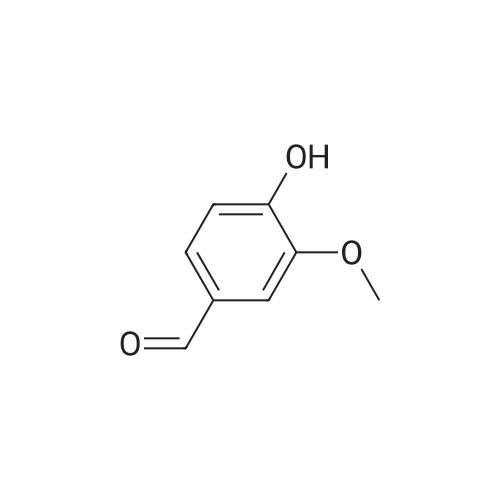
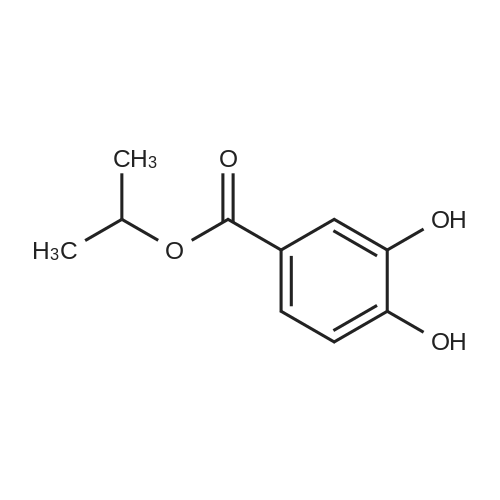
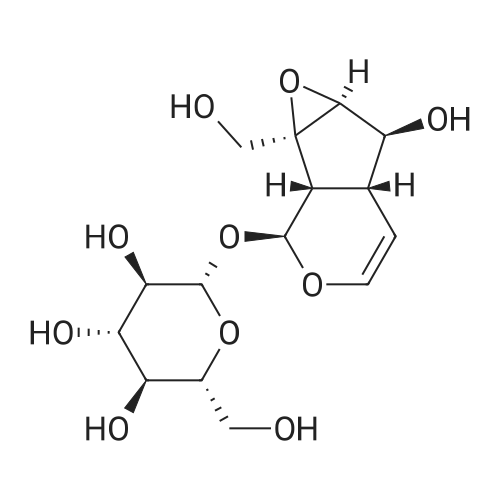
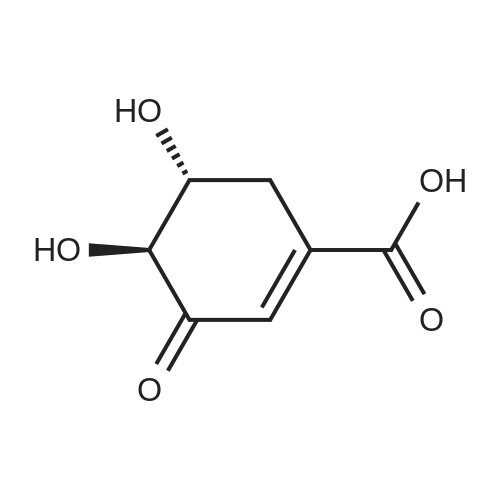
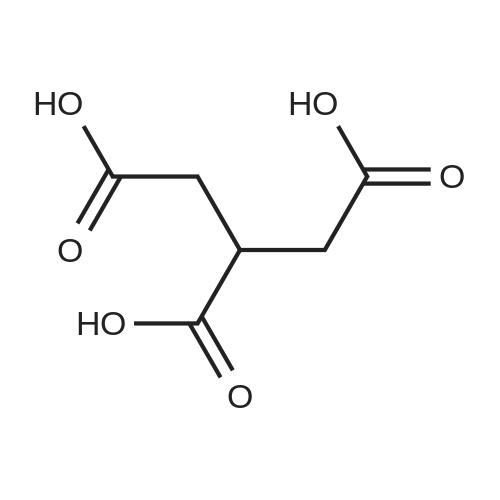
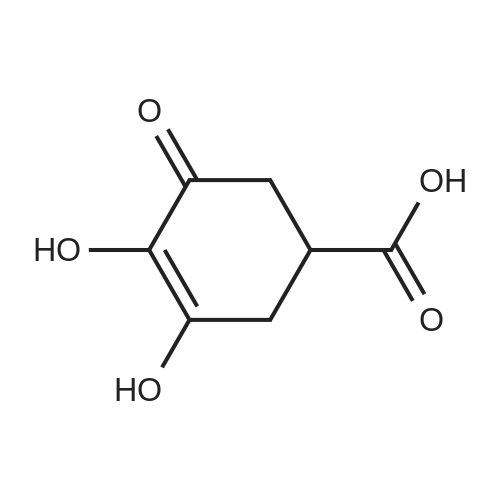
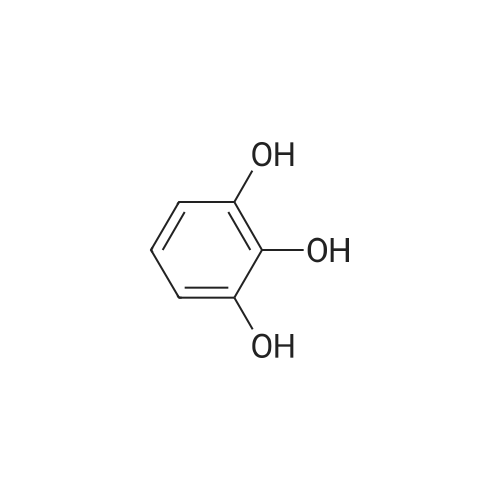
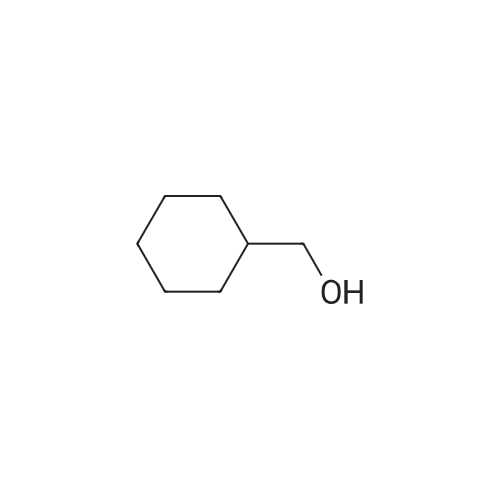
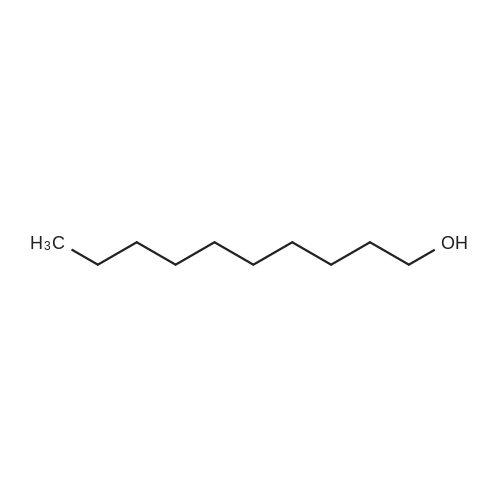
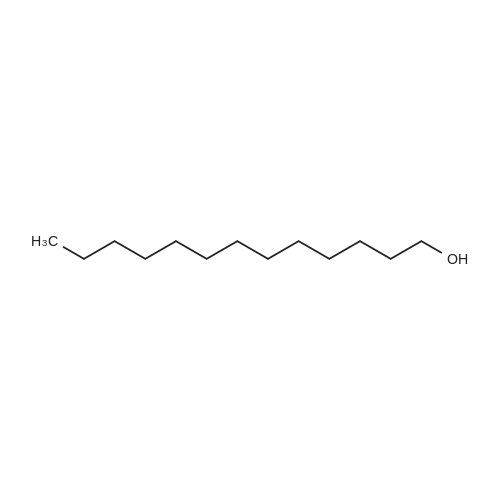
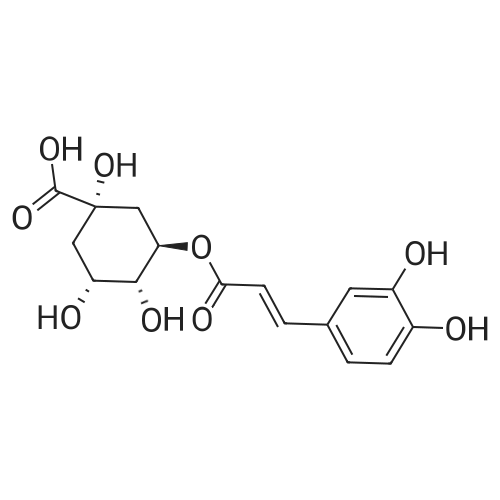
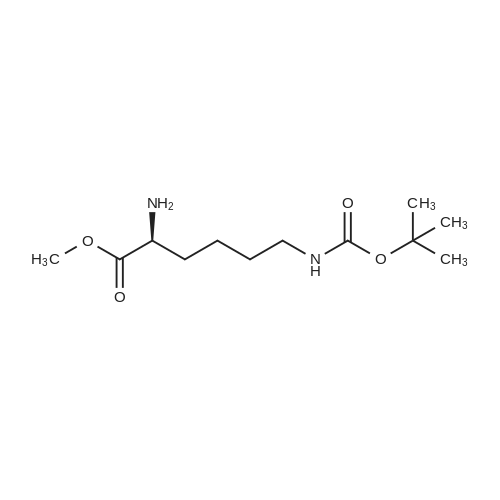
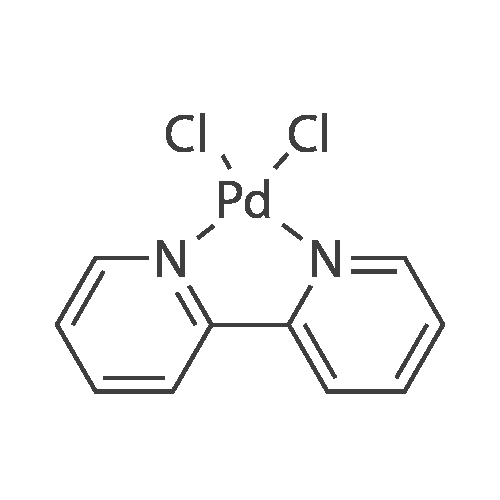
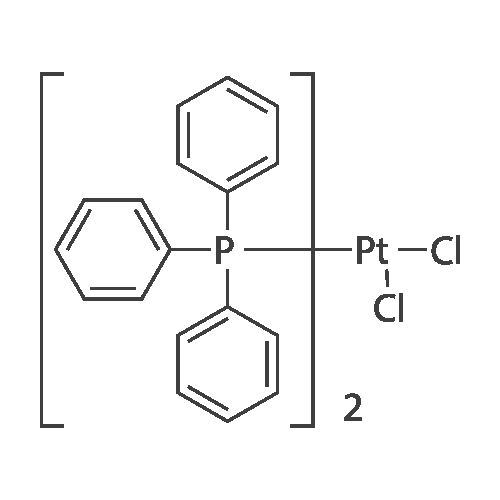
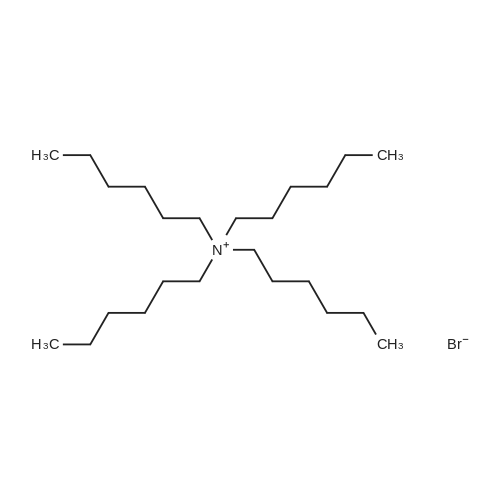
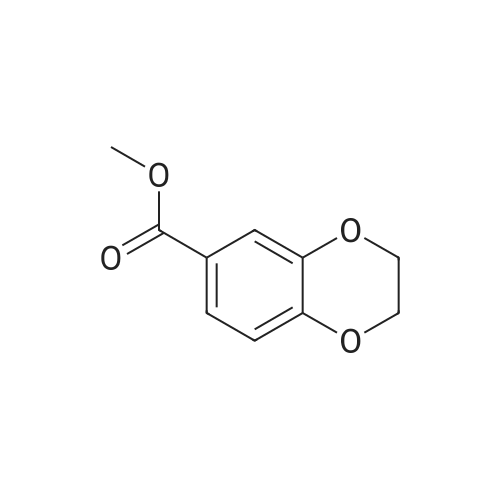
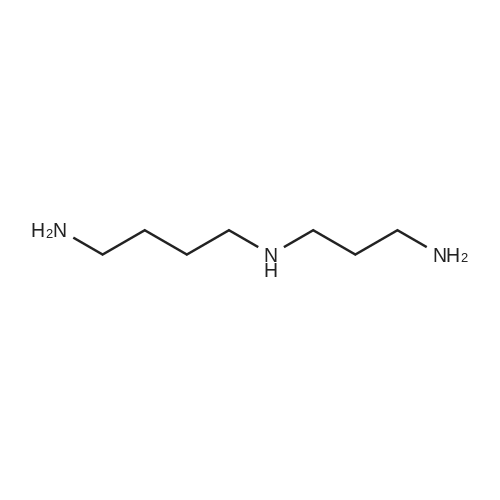
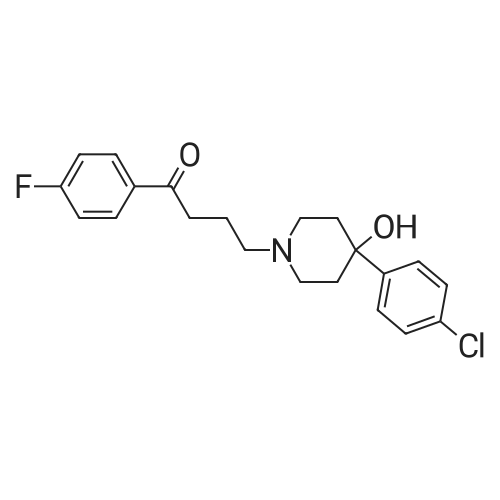
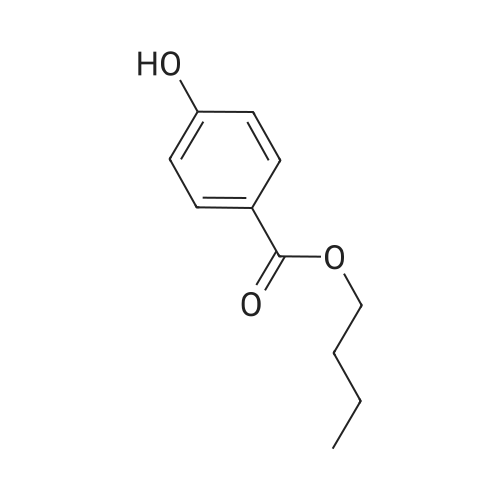
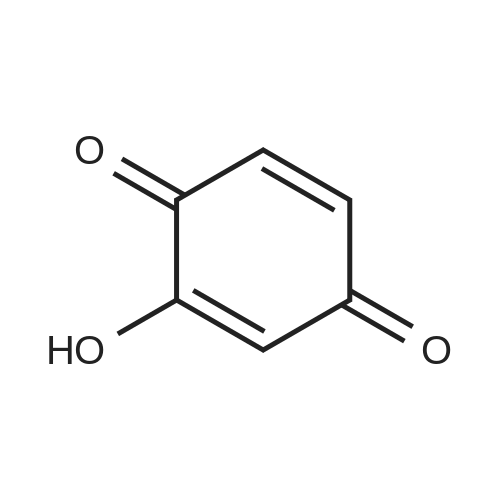
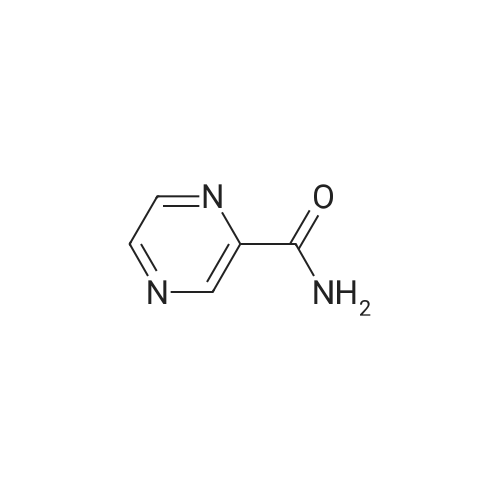
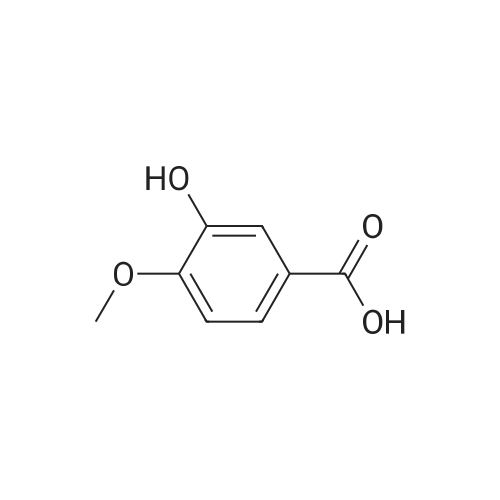
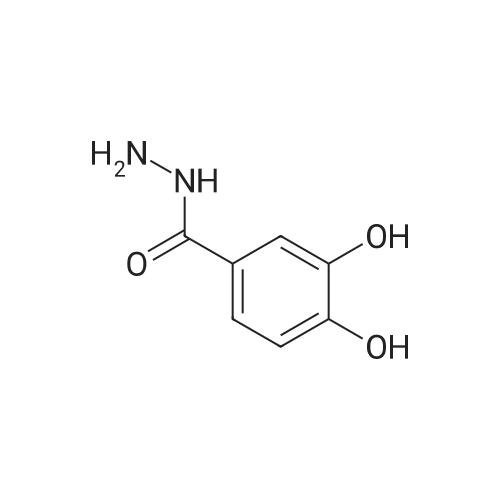
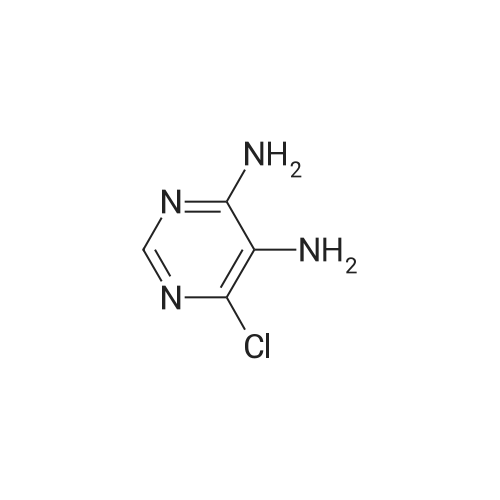
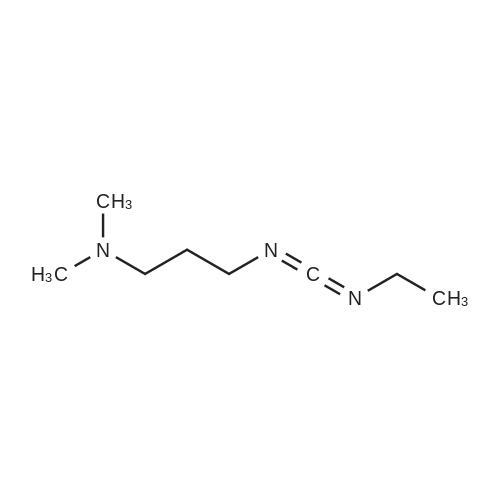
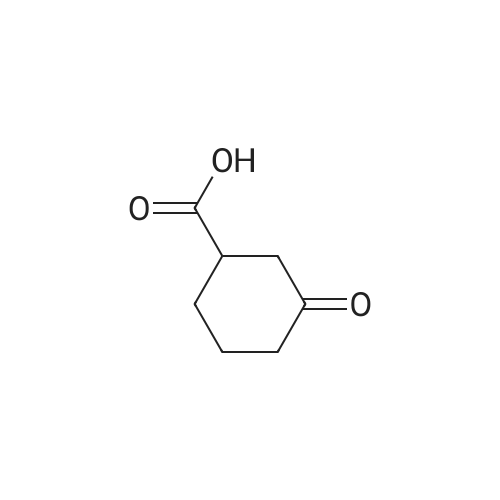
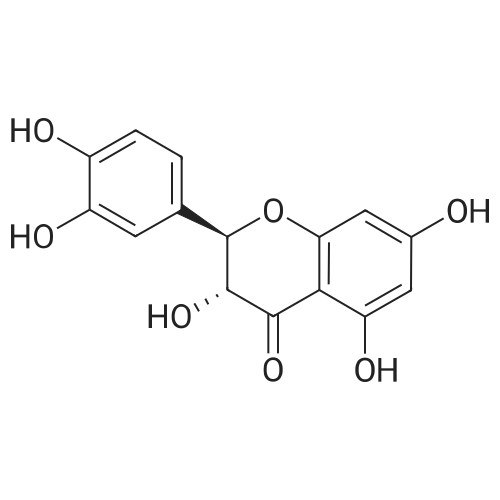
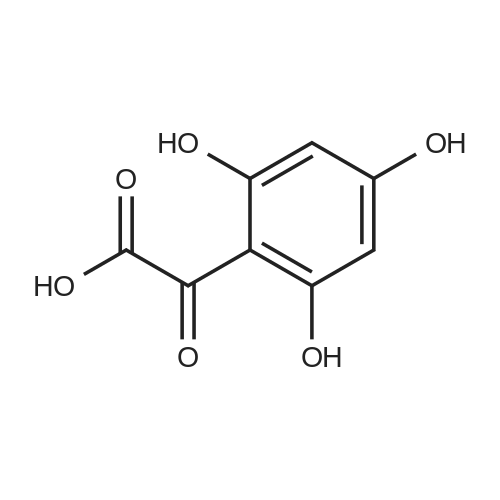
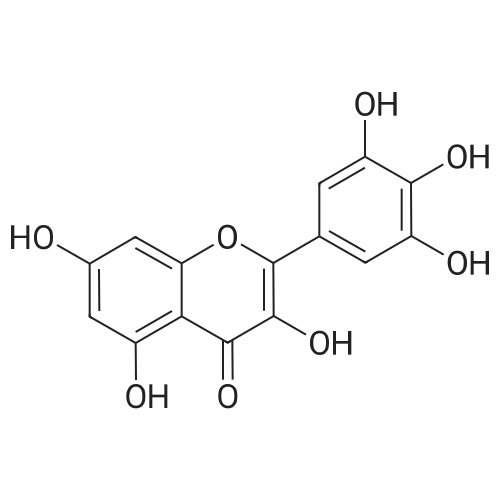
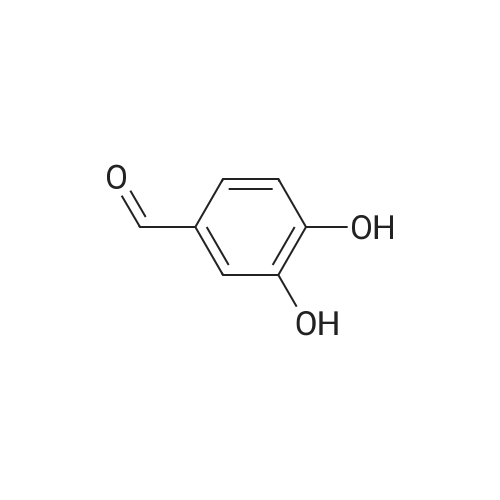
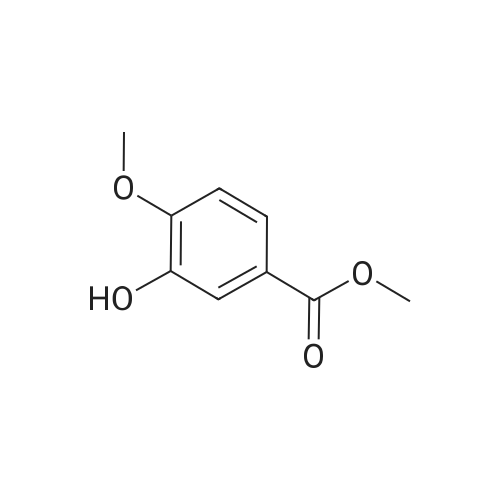
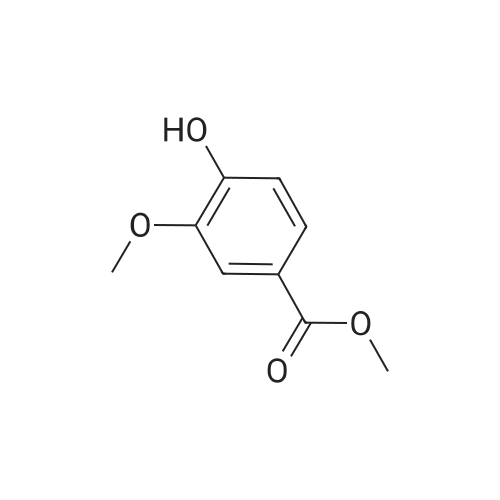
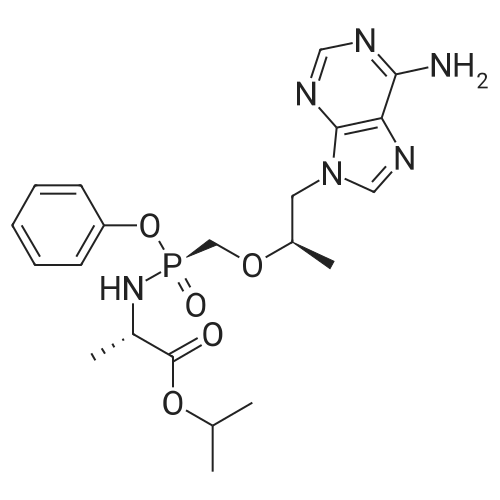
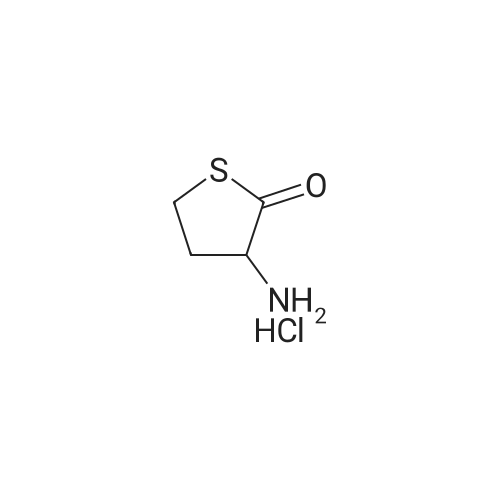
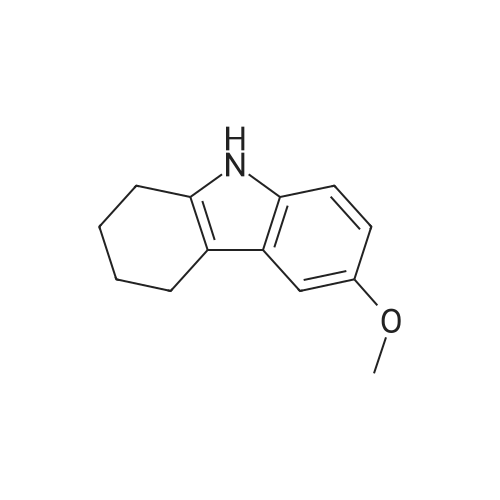
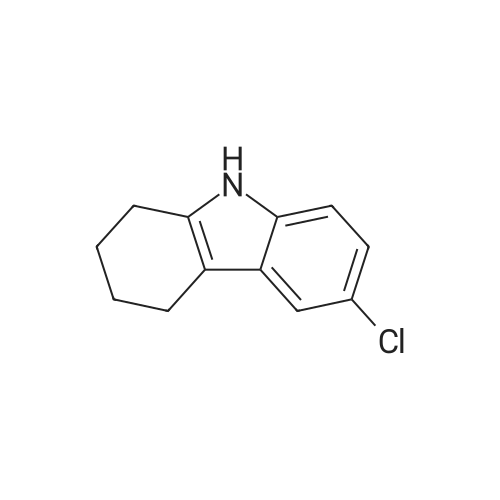
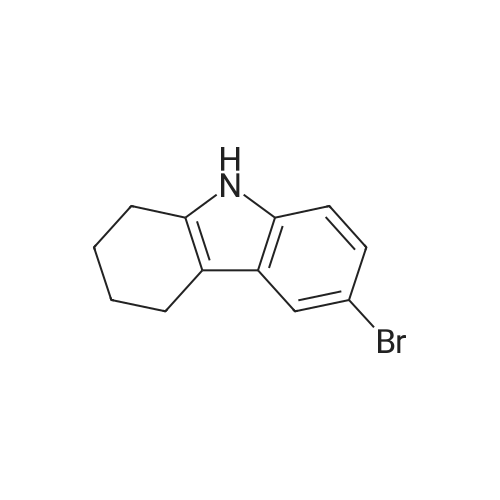
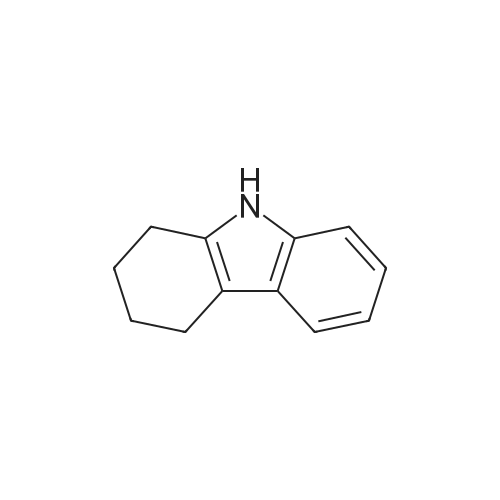
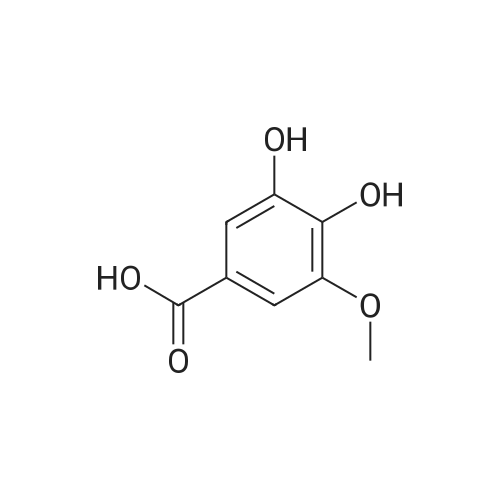
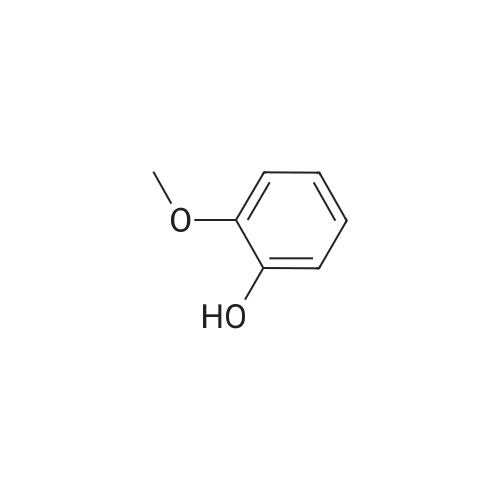
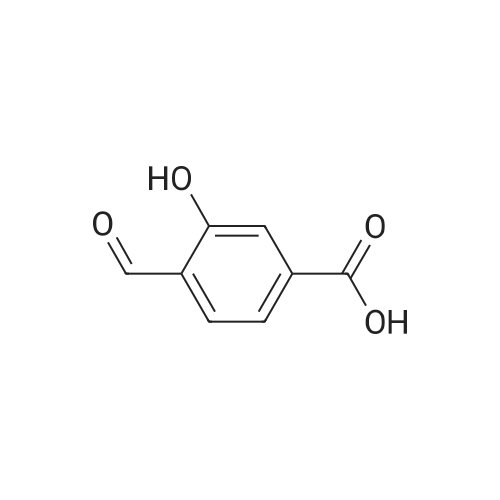
| 98% |
With sulfuric acid Heating; |
|
| 98% |
With sulfuric acid for 12h; Reflux; |
|
| 98% |
With sulfuric acid at 80℃; for 5h; |
1.1
To the mixture of 3,4-dihydrobenzoic acid (20 g, 129.7 mmol) in MeOH (260 mL) wasadded concentrated sulfuric acid (13.8 mL, 259.5 mmol). The reaction mixture wasstirred at 80 ° C for 5 hours. The resulting mixture was concentrated in vacuo. The crude product was washed with H2O and dried under high vacuum to give the titlecompound (21.5 g, 98%). |
| 98% |
With thionyl chloride at 0 - 20℃; for 16h; |
|
| 97% |
With thionyl chloride at 0 - 50℃; for 13h; Inert atmosphere; |
|
| 96% |
With sulfuric acid for 9h; Heating; |
|
| 96% |
With sulfuric acid for 4h; Reflux; |
2.1
A solution of 3,4-dihydroxybenzoic acid (5.0 g, 32 mmol) in methanol (80 mL) containing c-H2SO4 (3 mL) was heated at reflux for 4 hrs and cooled to room temperature. After concentrating under reduced pressure, the residue was dissolved in ethyl acetate and washed successively with water, saturated NaHCO3, and brine. The organic fraction was dried over MgSO4 and concentrated to give 3,4-dihydroxybenzoic acid methyl ester (5.2 g, 96%). 1H NMR (DMSO-(I6, 600 MHz) δ 9.77 (s, IH), 9.35 (s, IH), 7.35 (s, IH), 7.30 (d, IH, J=7.8 Hz), 6.79 (d, IH, J=7.8 Hz), 3.78 (s, 3H). |
| 96% |
With thionyl chloride at 20℃; for 12h; |
|
| 95% |
With thionyl chloride for 2h; Reflux; |
|
| 95% |
With thionyl chloride at 0 - 20℃; for 12h; Inert atmosphere; |
|
| 94% |
With sulfuric acid Heating; |
|
| 93% |
With toluene-4-sulfonic acid for 2h; Inert atmosphere; Reflux; |
1-1 Example 1-1 Synthesis of Methyl Protocatechuate
Under the protection of nitrogen gas, a magnet, 15.4g of protocatechuic acid (0.1mol) and 81mL of methanol were added to the dry three-necked flask.Then 77 mg of p-toluenesulfonic acid was added.Warm up to reflux temperature and react for 2 hours, cool to room temperature, distill to remove excess methanol,The remaining solid was recrystallized with water and dried in a vacuum drying oven to obtain methyl protocatechuate with a yield of 93%. |
| 93% |
With sulfuric acid for 6h; Reflux; |
|
| 91% |
With sulfuric acid at 65℃; for 24h; |
|
| 90% |
With hydrogenchloride at 80℃; for 12h; |
|
| 90% |
With thionyl chloride for 12h; Heating; |
|
| 90% |
With sulfuric acid for 8h; Reflux; |
Protocatechuic acid in accordance with the following procedure, was used by methyl esterification. Protocatechuic acid 14g, the methanol 50mL was mixed with 100mL eggplant type flask, was added to concentrated sulfuric acid 1mL, the mixture was heated and stirred under reflux for 8 hours. After the reaction, the methanol was evaporated and the resulting solid was dissolved in ethyl acetate, addition of ion-exchanged water, the aqueous layer was separated until neutral. Thereafter, the organic layer was separated, to give a solid by evaporation the organic layer. The solid was dried under reduced pressure to obtain methyl protocatechuic acid in 13.7g (90% yield). |
| 90.7% |
With sulfuric acid Heating; |
1.A; 2.A Step A: Methyl 3,4-dihydroxybenzoate (Intermediate 100B)
Sulfuric acid (138.0 mL, 2.60 mol, 2.0 eq.) was added to stirred a solution of 3,4-dihydroxy benzoic acid (100A) (200.0 g, 1.30 mol, 1.0 eq.) in methanol (2.0 L). The reaction mixture was stirred at 80° C. for 15 h. The progress of the reaction was monitored by TLC (50% EtOAc in n-hexane). The solvent was removed by distillation under vacuum then DMW (1500 mL) was added and the product was extracted with ethyl acetate (3*1.5 L). The combined organic extract was washed with saturated solution of sodium bicarbonate (2000 mL) brine (1500 mL), dried over sodium sulfate and concentrated under vacuum to obtained the intermediate 100B (198.0 g, 90.70%) as an off white solid. 1H NMR (300 MHz, DMSO): δ 9.78 ppm (s, 1H), 9.37 ppm (s, 1H), 7.36-7.23 ppm (m, 2H), 6.81 ppm (d, J=8.2 Hz, 1H), 3.76 ppm (s, 3H). |
| 88% |
With sulfuric acid In tetrahydrofuran at 45 - 65℃; for 120 - 240h; Molecular sieve; Darkness; |
4.1 Chemical synthesis
General procedure: PA esters were synthesized as described by Reis et al. (2010). Modifications to the general procedure were made to allow the use of fatty alcohols with a longer carbon chain and facilitate the purification step. Briefly, 1 g (6.49 mmol) of protocatechuic acid was solubilized in 3 mL of THF. Then, 3 M equivalents (19.47 mmol) of the corresponding alcohol (from methanol to octadecanol, C1 to C18, respectively) were added. Sulfuric acid was used as catalyst (4%, v/v of the final reaction mixture volume) and the reaction mixtures were incubated in an orbital shaker (250 rpm) at temperatures varying from 45 to 65 °C, depending on the alcohol used, for approximately 5-10 days, protected from light. To remove water generated during the reaction, molecular sieves were added to the medium (40 mg/mL of final volume). At different time intervals, samples were withdrawn from the reaction mixture, and then analyzed by reversed-phase HPLC (Section 2.3). The reaction was stopped by the addition of 2 mL of sodium carbonate (1 M). The mixture was dissolved in approximately 100 mL of acetonitrile and filtrated (150 mm standard pleated filter, Grosseron, Saint-Herblain, France). The resulting solution was stocked at 4 °C until purification. In the case of the esters with an alkyl chain length from 14 to 18 carbons (solids at room temperature), it was necessary to wash the filter using heated acetonitrile. |
| 86.5% |
With hydrogenchloride In lithium hydroxide monohydrate for 12h; |
11.A
Example 11; 3,4-dihydroxy- N-(2-oxo-2,3-dihydro-l H-benzo [d]imidazol-5-yl)benzamide (referred to as DC-0051-B3).; 3,4-Dihydroxybenzoic acid was converted to its methyl ester by refluxing in methanol in presence of acid. The dihydroxy group was protected as its benzyl ether by treating with benzyl bromide and potassium carbonate. Hydrolysis of the ester using sodium hydroxide provided the acid which was coupled to 5-amino-2,3- dihydro-1H-benzoimidazol-5-one using N, N-1,3-diisopropylcarbodiimide in presence of 1-hydroxybenzotriazole to provide the amide. The amide was debenzylated by hydrogenation in presence of palladium on carbon. A) 3, 4-Dihydroxy benzoic acid methyl ester; A solution of 3, 4-dihydroxy benzoic acid (2.8 g) in methanol (150 ml) was refluxed in presence of concentrated hydrochloric acid (0.5 ml) for 12 hrs. After concentrating under reduced pressure, the residue was dissolved in ethyl acetate (150 ml) and washed with water (50 ml) 10 % sodium bicarbonate solution (50 ml), brine solution (50 ml) and dried over anhydrous magnesium sulfate. Removal of the solvent under reduced pressure provided 2.64 g of 3,4-dihydroxybenzoic acid methyl ester. (Yield = 86.5 %). 'H NMR CDC13 7.7 (1H, s) 7.63 (1H, d, J 8Hz) 6.92 (1H, d, J 8Hz) 5.7 (2H, 3.92 (3H, s) |
| 86% |
With hydrogenchloride Inert atmosphere; |
|
| 85% |
With toluene-4-sulfonic acid for 72h; Heating; |
|
| 85% |
With thionyl chloride at 0℃; Reflux; Inert atmosphere; |
5.2. General procedure for preparation of esters
General procedure: Thionyl chloride (1.16 g, 1.5 equiv) was added drop-wise to a solution of acid (1.0 g, 1.0 equiv) in the corresponding alcohol (15 ml) at 0&d eg;C. The solution was refluxed under a nitrogen atmosphere until all starting material was consumed (TLC monitoring). Then the solvent was removed under vacuo and the residue was purified by silica gel column chromatography eluting with ethyl acetate/n-hexane to afford the corresponding carboxylic esters. |
| 82% |
With sulfuric acid for 18h; Reflux; |
|
| 81% |
With sulfuric acid at 20℃; for 120h; |
|
| 80% |
With sulfuric acid Reflux; |
|
| 76% |
With hydrogenchloride In lithium hydroxide monohydrate at 80℃; |
|
| 73% |
With sulfuric acid for 168h; Heating; |
|
| 24% |
With sulfuric acid for 24h; Reflux; |
|
|
With hydrogenchloride |
|
|
With hydrogenchloride |
|
| 3.5 g |
With sulfuric acid for 5h; Heating; |
|
|
With sulfuric acid |
|
|
With trichlorophosphate Heating; |
|
|
With hydrogenchloride at 20℃; |
|
|
With sulfuric acid |
|
|
With sulfuric acid Heating; |
|
|
With sulfuric acid |
102 3,4-Dihydroxy benzoic acid methyl ester
EXAMPLE 102 3,4-Dihydroxy benzoic acid methyl ester A mixture of 100 g of 3,4-dihydroxy benzoic acid, 600 ml methyl alcohol and 10 ml of concentrated sulfuric acid is heated at reflux for 18 hours. The reaction mixture is cooled to room temperature, poured into water and extracted with methylene chloride. The organic layer is washed with saturated sodium bicarbonate and sodium chloride, dried and concentrated in vacuo to give 50.6 g of the desired product. MP 130°-131° C. EI-MS:m/z 168 (M+). |
|
With hydrogenchloride for 4h; Reflux; |
|
|
With sulfuric acid Reflux; |
|
|
With sulfuric acid |
|
|
With sulfuric acid at 40℃; for 2h; |
|
|
With dicyclohexyl-carbodiimide In 1,4-dioxane at 5℃; for 48h; |
|
|
With sulfuric acid at 90℃; for 8h; |
General procedure for the preparation of compounds 2.
Protocatechuic acid (1 mmol) in methanol (30 ml) was treated with concentrated sulfuric acid (0.5 ml) under 90 oC overnight. The sovlent was removed leaving oil which was dissovled in ethyl acetate (20 ml) and extracted with water (40 ml). After drying the organic layer with anhydrous Na2SO4 and evaporating the solvent under reduced pressure a solid appeared. The solid was recyrstallized from ethanol to obtain the compound 2. |
|
With sulfuric acid Reflux; |
|
|
With thionyl chloride at 0 - 50℃; for 8h; |
|
|
With thionyl chloride at 20℃; for 8h; |
|
|
With sulfuric acid at 90℃; for 8h; |
4.1.1 General procedure for the preparation of compound 2
Protocatechuic acid (1mmol) in methanol (30mL) was treated with concentrated sulfuric acid (0.5mL) under 90°C overnight. The sovlent was removed leaving oil which was dissolved in ethyl acetate (20mL) and extracted with water (40mL). After drying the organic layer with anhydrous Na2SO4 and evaporating the solvent under reduced pressure a solid appeared. The solid was recrystallized from ethanol to obtain the compound 2. |
|
With sulfuric acid for 8h; Reflux; |
|
|
With sulfuric acid Reflux; |
1.1 example 1
(1) the 3,4-dihydroxy benzoic acid, concentrated sulfuric acid, methanol reflux reaction, TLC detection until the raw material disappeared, the obtained solid product; 3,4-dihydroxybenzoic acid, the amount of the concentrated sulfuric acid and methanol is the proportion of 1 : 0.1: 5, wherein 3,4-dihydroxy benzoic acid to mmol meter, concentrated sulfuric acid, in terms of ml of methanol. |
|
With thionyl chloride at 60℃; for 10h; Inert atmosphere; |
3,4-Dibenzyloxybenzoic acid (9b)
To a solution of 8b (15.0 g, 97.4 mmol) in MeOH (360 mL) was added SOCl2 (7.9 mL, 48.7 mmol) fordropwise at 60 °C. The reaction mixture was stirred at 60 °C for 10 h. Then, the reaction mixture wasevaporated under reduced pressure. The crude mixture was applied to following reaction without furtherpurification.To a mixture of crude residue and K2CO3 (40.4 g, 0.292 mol) in DMF (162 mL) was added benzylbromide (29.0 mL, 0.244 mol) at 100 °C. The reaction mixture was stirred at 100 °C for 16 h. Then, thereaction mixture was filtered through a pad of Celite, diluted with H2O, and extracted with EtOAc. Theorganic layer was washed with water and brine, dried over anhydrous MgSO4, and evaporated underreduced pressure. The crude mixture was applied to following reaction without further purification.To a stirred solution of crude residue in MeOH/THF (1/1, 324 mL) were added 4 M NaOH aq. (85 mL,0.34 mol) at 80 °C. The reaction mixture was stirred at same temperature for 1 h. Then, the reactionmixture was quenched with 6 M HCl aq., filtered and washed with H2O to give 9b (32.2 g, 96.3 mmol,99%) as a white solid.Spectral data for 9b were in good agreement with those reported in reference.16 |
|
Heating; |
4.1.1. General procedure for the preparation of methyl 3,4-dihydroxybenzoate(1)
To a stirred solution of 3,4-dihydroxybenzoic acid (1 mmol) inmethanol (30 mL) was added thionyl chloride (1 mL) dropwiseover 1 h at 0 C. The mixture was further stirred overnight at50 C. The solution was cooled to room temperature, and dilutedwith water (25 mL). Excess methanol was distilled out and the pH was adjusted to 6 with saturation sodium bicarbonate. Then,the mixture was extracted with ethyl acetate and washed withbrine. After drying the organic layer with anhydrous sodium sulfateand evaporating the solvent under reduced pressure a solidappeared. The crude product was recrystallized from ethanol togive compound 1. The completion of the reaction was monitoredon TLC by using and ethyl acetate petroleum ether (1:1) andobserved in UV light. |
|
With sulfuric acid at 63℃; |
Synthesis of the ligand.
Protocatechuic acid (0.0973 mol, 15 g) was dissolved in methanol (100 ml) with stirring,catalyzed by concentrated sulfuric acid. The mixture was stirred for about 20 h at 63°C and evaporated. The concentratedsolution was poured into 200 ml of ethyl acetate and 100 ml of water and stilled. The ethyl acetate solvent was evaporated togive white powdered compound 1. |
|
With thionyl chloride |
|
|
With thionyl chloride for 1h; Reflux; |
Synthesis of compounds 7a-7e
General procedure: In a dry round-bottomed flask, protocatechuic acid (2 mmol, 0.308 g) and the respective alcohol(MeOH, n-propanol, n-butanol, n-pentanol, or n-hexanol-10 mL) were added. Immediately,2 mL of SOCl2 were added dropwise. The system was kept under reflux for 1 h and thevolatiles were evaporated under reduced pressure. The residue was dissolved in 25 mL ofEtOAc, washed with 2 x 15 mL aqueous NaHCO3, and with 3 x 25 mL of H2O. The organicphase was dried with anhydrous Na2SO4 and partially evaporated. The crude material waspurified over silica gel column chromatography eluted with n-hexane:EtOAc 1:1 to afford 7a-7e (for NMR and ESI-HRMS spectral data, see S1 File). |
|
With sulfuric acid at 100℃; for 2h; |
2.1 Step 1.
General procedure: Add compound 1a (18mmol) to 19mL methanol MeOH solution, stir, and then add 0.45mL H2SO4 with a mass fraction of 98.3%. The reaction mixture is refluxed at 100°C for 2 hours, distilled under reduced pressure, and the residue is dissolved in water , And extracted with dichloromethane (DCM), the organic layer was washed with saturated brine, dried over anhydrous Na2SO4, filtered, and the filtrate was concentrated under reduced pressure to obtain compound 1b (2.68g); |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













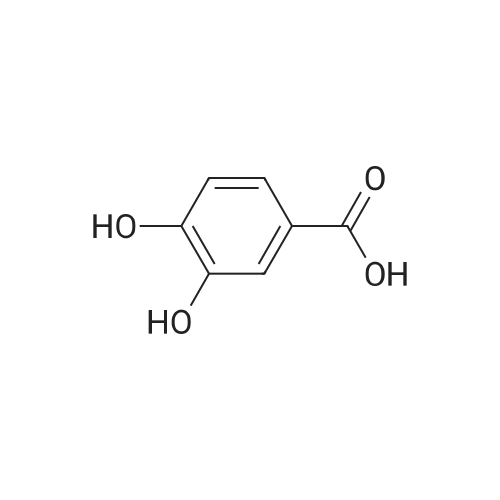

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping