| 20.66 g |
With lithium hexamethyldisilazane In tetrahydrofuran at 20 - 30℃; for 3.46667h; |
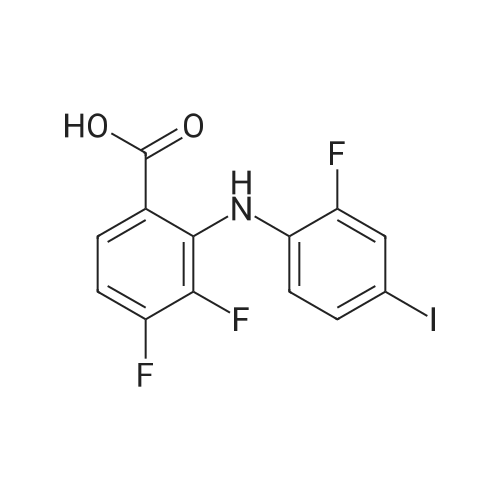
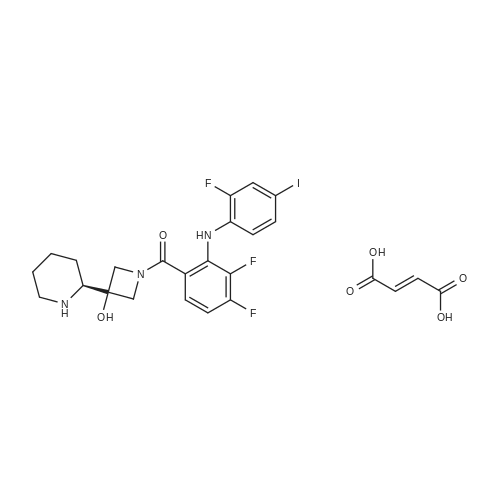
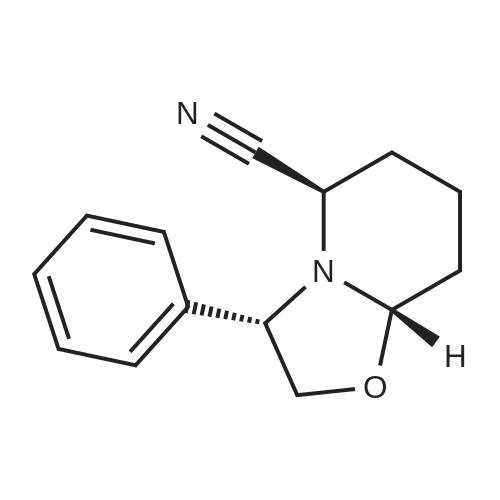
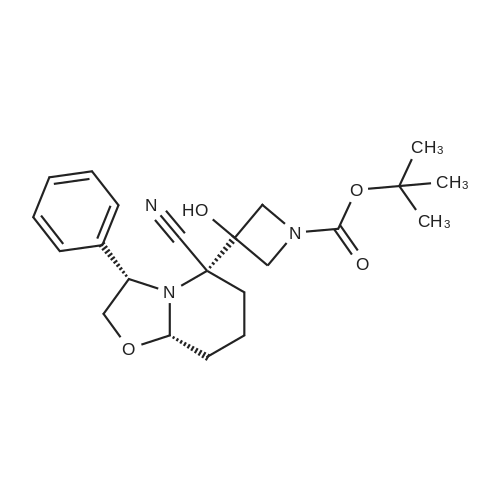
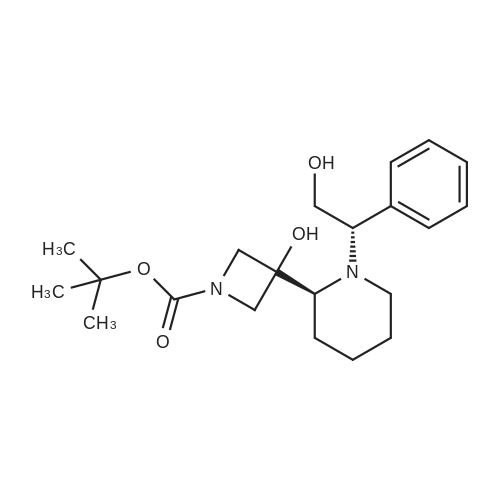
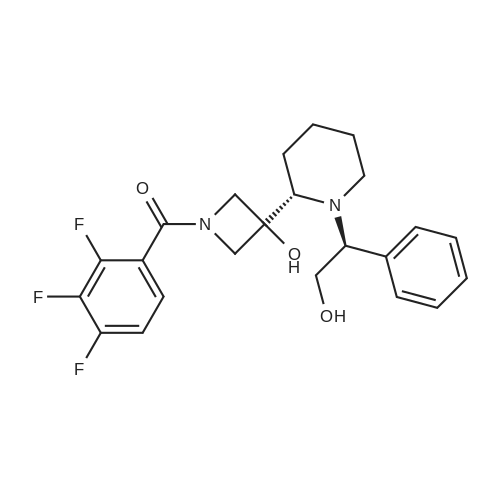
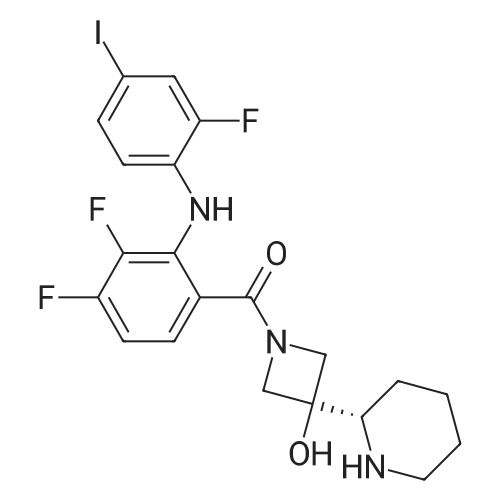
6 Example6Synthesis of [3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-phenyl]-((S)-3-hydroxy-3-piperidin-2-yl-azetidin-1-yl)-methanone
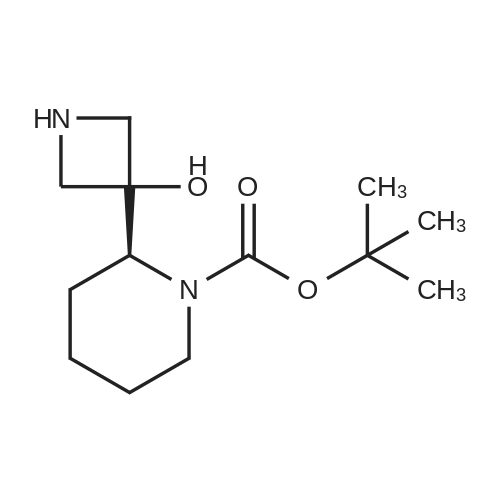
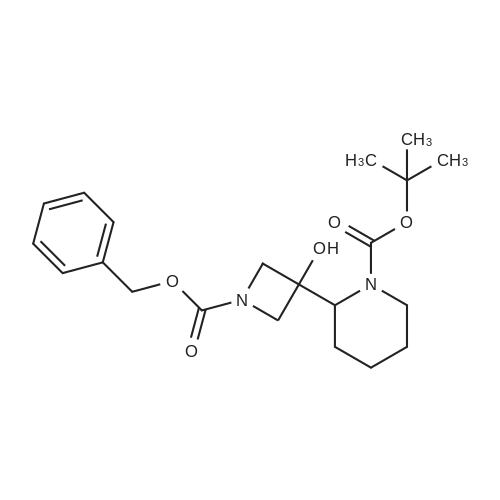
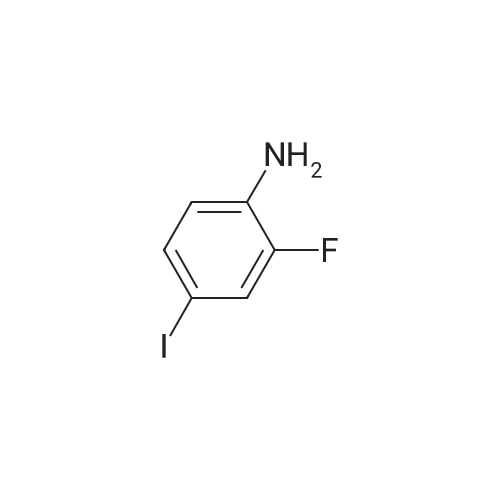
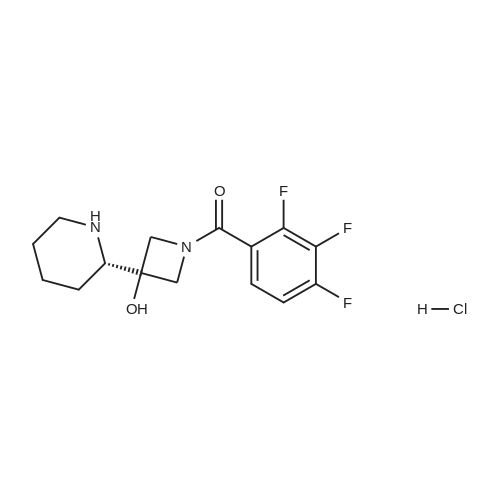
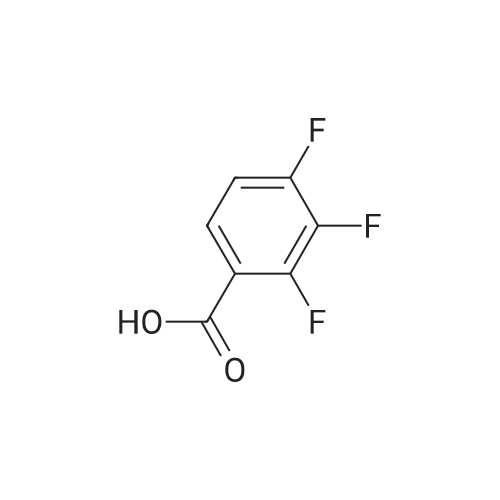
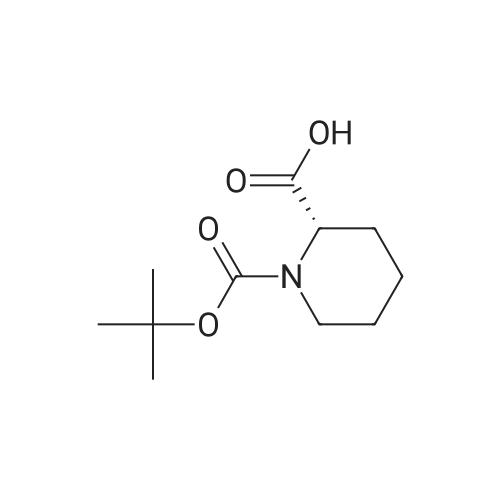
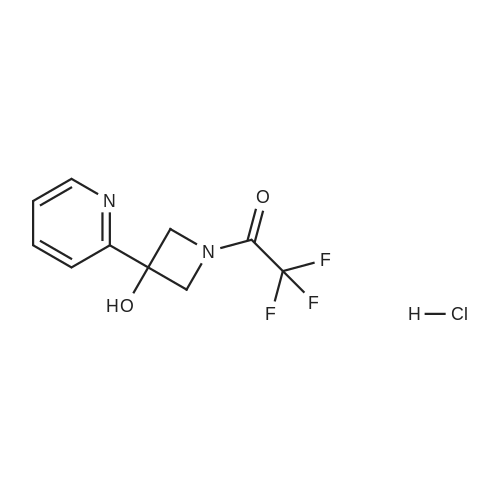
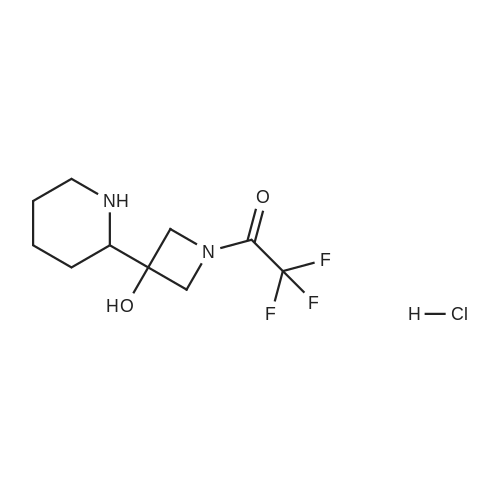
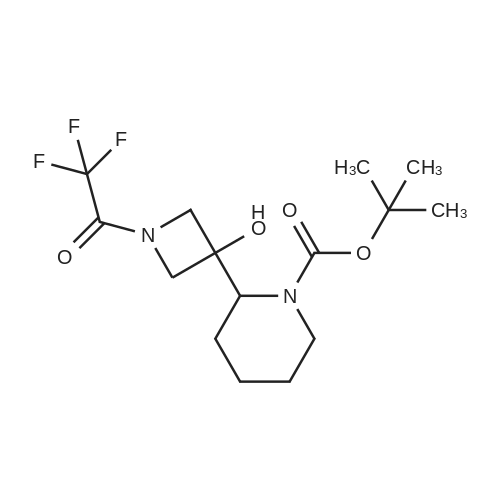
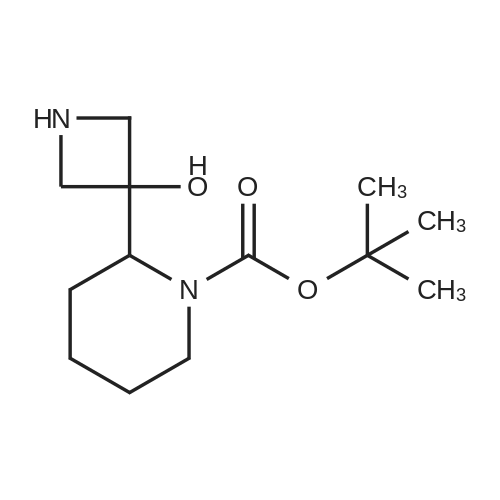
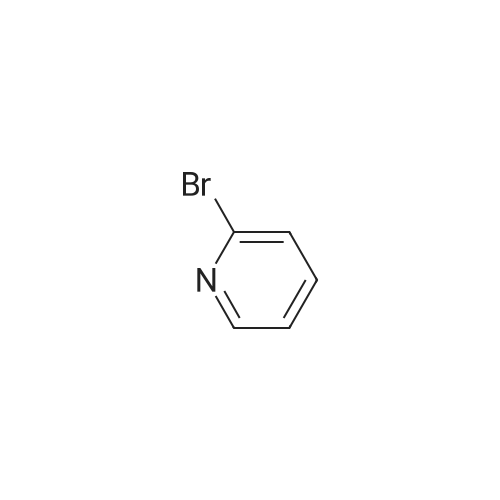
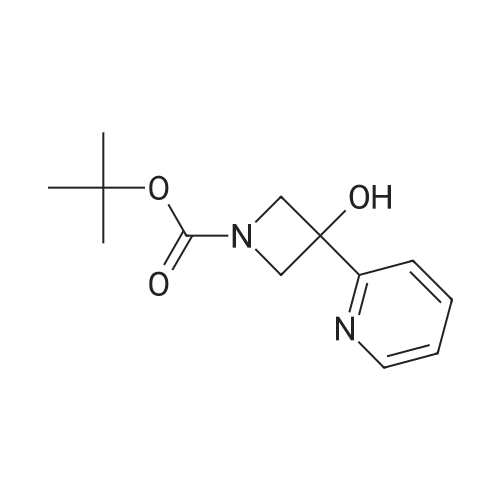
To a solution of ((S)-3-hydroxy-3-piperidin-2-yl-azetidin-1-yl)-(2,3,4-trifluorophenyl)-methanone hydrochloride (15.0 g,42.8 mmol, 1.0 eq.) and 2-flouro-4-iodo-anilin (11.1 g,47 mmol, 1.1 eq.) in THF (90 ml), a solution ofLiHMDS in THF (149 g, 20.7% w/w, 184 mmol,4.3 eq.) was dosed over 88 min at 20 to 30 °C. Stirring was continued for 2 h. After completeconversion, the mixture was dosed to a mixture of sulfuric acid (12.0 g, 96%-w/w, 118 mmol,2.75 eq.) in water (75 mL) over 25 min and kept stirring for 1 h. The layers were allowed toseparate, and the organic phase was washed with a mixture of water ( 60 mL) and toluene (96mL ). The organic phase was concentrated under vacuum to a volume of approximately 150 mL.Toluene (250 mL) was added and residual THF was removed by distillation at 55 oc jackettemperature and at a pressure of 84 mbar while keeping the batch volume constant by continuousdosing of toluene ( 400 mL ), resulting in slow precipitation of the product. The batchtemperature was then lowered to 10 oc within 2 h, and the suspension was kept stirring overnightat 10 °C. The product was filtered off, and the cake was rinsed with cold toluene (150 mL).Drying overnight under vacuum at 35 °C until weight constancy yielded the title compound(20.66 g) as a colorless product. HPLC purity: 99.7%-area. M.p (DSC): Tonset: 166.7°C,extrapolated peak: 168.2°C (91.5 J/g). 1H NMR (600 MHz, CDCh): o 8.28- 8.48 (br, 1 H), 7.39(dd, 1 H), 7.32 (ddd, 1 H), 7.09-7.14 (m, 1 H), 6.75-6.86 (br, 1 H), 6.60 (ddd, 1 H), 4.10 (d, 2H), 4.05-4.20 (br, 1 H), 3.93- 4.04 (br, 1 H), 3.09 (d, 1 H), 2.70 (d, 1 H), 2.56- 2.67 (br, 1 H),1.68 - 1.87 (m, 1 H), 1.50 - 1.64 (m, 2 H), 1.25 - 1.38 (m, 2 H), 1.07 - 1.24 (m, 1 H). MS (EI): mlz = 532 ([M+Ht, 100%). EA for C21H21F3IN203: calcd: C 47.47, H 3.98, N 7.91, F 10.73;found C 47.68, H 4.00, N 7.66, F 10.80. |
| 20.66 g |
With lithium hexamethyldisilazane In tetrahydrofuran at 20 - 30℃; for 3.46667h; |
6 Example 6
Synthesis of (S)-[3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl][3-hydroxy-3-(piperidin-2-yl)azetidin-1-yl]methanone (Compound I)
To a solution of ((S)-3-hydroxy-3-piperidin-2-yl-azetidin-l-yl)-(2,3,4-trifluoro- phenyl)-methanone hydrochloride (15.0 g,42.8 mmol, 1.0 eq.) and 2-flouro-4-iodo-anilin (11.1 g, 47 mmol, 1.1 eq.) in THF (90 ml), a solution of LiHMDS in THF (149 g, 20.7% w/w, 184 mmol, 4.3 eq.) was dosed over 88 min at 20 to 30 °C. Stirring was continued for 2 h. After complete conversion, the mixture was dosed to a mixture of sulfuric acid (12.0 g, 96%-w/w, 118 mmol, 2.75 eq.) in water (75 mL) over 25 min and kept stirring for 1 h. The layers were allowed to separate, and the organic phase was washed with a mixture of water (60 mL) and toluene (96 mL). The organic phase was concentrated under vacuum to a volume of approximately 150 mL. Toluene (250 mL) was added and residual THF was removed by distillation at 55 °C jacket temperature and at a pressure of 84 mbar while keeping the batch volume constant by continuous dosing of toluene (400 mL), resulting in slow precipitation of the product. The batch temperature was then lowered to 10 °C within 2 h, and the suspension was kept stirring overnight at 10 °C. The product was filtered off, and the cake was rinsed with cold toluene (150 mL). Drying overnight under vacuum at 35 °C until weight constancy yielded the title compound (20.66 g) as a colorless product. HPLC purity: 99.7%-area. M.p (DSC): TonSet: 166.7°C, extrapolated peak: 168.2°C (91.5 J/g). NMR (600 MHz, CDC13): δ 8.28 - 8.48 (br, 1 H), 7.39 (dd, 1 H), 7.32 (ddd, 1 H), 7.09 - 7.14 (m, 1 H), 6.75 - 6.86 (br, 1 H), 6.60 (ddd, 1 H), 4.10 (d, 2 H), 4.05 - 4.20 (br, 1 H), 3.93 - 4.04 (br, 1 H), 3.09 (d, 1 H), 2.70 (d, 1 H), 2.56 - 2.67 (br, 1 H), 1.68 - 1.87 (m, 1 H), 1.50 - 1.64 (m, 2 H), 1.25 - 1.38 (m, 2 H), 1.07 - 1.24 (m, 1 H). MS (EI): m/z = 532 ([M+H]+, 100%). EA for C2,H2iF3rN203: calcd: C 47.47, H 3.98, N 7.91, F 10.73; found C 47.68, H 4.00, N 7.66, F 10.80. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping