| 87% |
With ammonium sulfate; at 115 - 135℃; for 16h;Industry scale; |
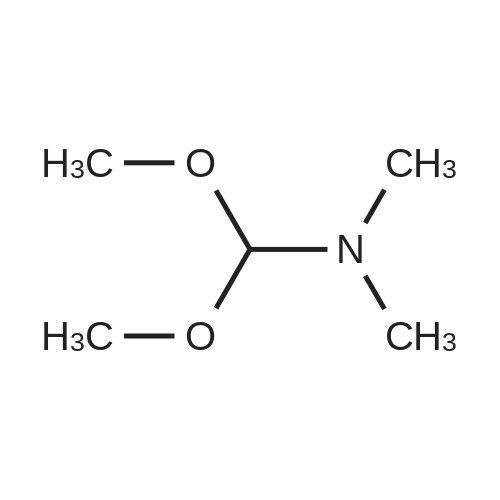
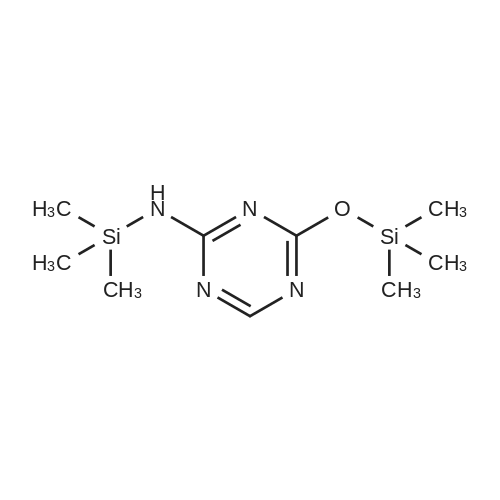
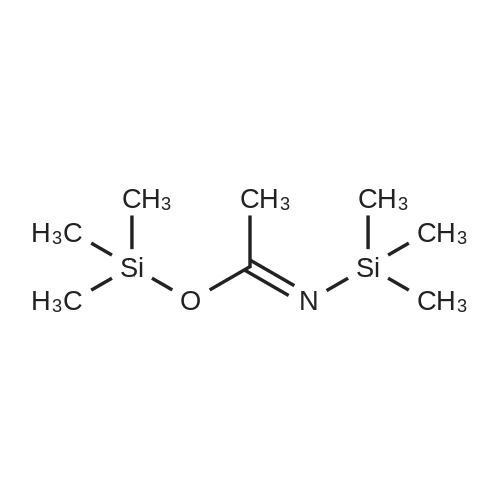
Example 1 Preparation of 2-[(trimethylsilyl)amino]-4-[(trimethylsilyl)oxy]-s-triazine(silyl <strong>[931-86-2]5-azacytosine</strong>) A mixture of <strong>[931-86-2]5-azacytosine</strong> (7.33 Kg), HMDS (33.9 Kg) and ammonium sulfate (0.44 Kg) was heated at reflux (about 115-135 C.) and stirred for 16 hours. After the reaction was complete, the slurry was cooled to 118 C. and then filtered through a bed of celite and rinsed with HMDS (5.6 Kg)(5.6 Kg). The silylated <strong>[931-86-2]5-azacytosine</strong> solution was cooled to 35 C. and the solution was cooled to 18 C., stirred at 18 C., for not less than 6.5 hours and then filtered. The solid was washed twice with HMDS (5.6 Kg each) and dried under vacuum at <=70 C. for 9.5 hours to obtain 14.19 Kg of white silyl <strong>[931-86-2]5-azacytosine</strong> (87%). |
|
With ammonium sulfate; for 8h;Heating / reflux; |
In a 22 L, 3-necked flask, a mixture of <strong>[931-86-2]5-azacytosine</strong> (1) (2.0 kg, 17.8 mol, 1.07 molar eq.), HMDS (9.162 kg) and ammonium sulfate (40.0 g) was heated at reflux for 2 hours. A fresh amount of ammonium sulfate (20.0 g) was added, and the reflux was continued for 6 hours longer. The initial slurry turned into a clear, pale-yellow, solution and no more gas evolved at the end of the reflux. The excess HMDS was evaporated off in vacuum to obtain an off-white residue, which is trimethylsilylated <strong>[931-86-2]5-azacytosine</strong> (6). |
|
With ammonium sulfate; for 5h;Heating / reflux; |
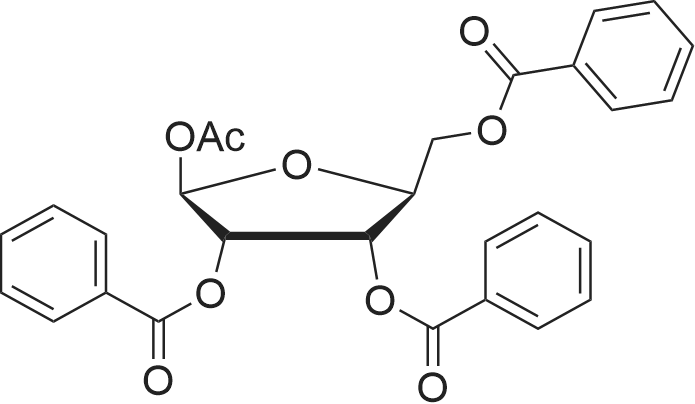
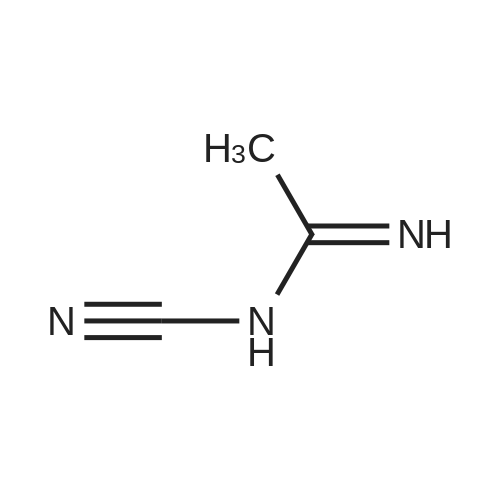
Reference Example 1 (Prior Art Preparation)[0056] This example demonstrates the preparation of 5-azacytidine according to prior art examples, e.g., Vorbrueggen et.al., J.Org.Chem. Vol. 39, No.25, 1974 and US Patent No. 7,038,038.[0057] 5-Azacytosine (200 g, 1.8 mol) was mixed with 1,1,1,3,3,3-hexamethyldisilazane (HMDS) (800 ml, 619.36 g, 3.837 mol) and ammonium sulfate (NH4^SO4 (5 g, 37.8 mmol). The resulting mixture was heated to reflux for a period of 5 hours. Then, the <n="17"/>mixture was cooled to 6O0C, and the excess HMDS was distilled off under reduced pressure. The residue was heated to 1350C for 30 minutes, and the product was cooled to ambient temperature to afford bis(trimethylsilyl)-<strong>[931-86-2]5-azacytosine</strong> (404 g, 1.58 mol). The <strong>[931-86-2]5-azacytosine</strong> was dissolved in dry 1,2-dichloroethane (125 ml), and 1,2,3, 5-tetra-O-acetyl-beta-D- ribofuranose (47 g, 0.1476 mol) was added. The reaction mixture was cooled to 5-1O0C and a solution of SnCl4 (42.18 g, 0.162 mol) in 1,2-dichloroethane (25 ml) was added dropwise over 15 minutes. The resulting mixture was stirred for 2 hours, during which time the temperature was allowed to reach ambient temperature. Sodium bicarbonate (NaHCO3) (70 g) was added under constant mixing and the reaction mixture was cooled to 150C. Purified water (140 ml) was added drop wise and mixing was maintained for additional 20 minutes, then 1,2-dichloroethane was added and mixing was maintained for 10 additional minutes. The organic and aqueous phases were separated, and the organic phase was filtered through a layer of Celite, washed with 1,2-dichloroethane, and dried over sodium sulfate (Na2SO4).[0058] The organic solvent was evaporated, and the residue was dissolved in methanol (120 ml), then heated to 6O0C to afford a clear solution. Charcoal (1.6 g) was added and the resulting mixture was stirred for 2 hours at ambient temperature. The charcoal was filtered off, and methanol/ammonia solution (200 ml of a 16% solution) was added to the filtrate and stirring was maintained for 20 hours at ambient temperature, during which time the reaction mixture solution gradually became viscous. Vacuum was applied to remove the excess ammonia, and the reaction mixture was cooled to 50C. The resulting solid was filtered off, washed with methanol (3 X 30 ml) and dried to obtain crude 5-azacytidine (8 g, 21% yield) having purity of 98.7% (according to HPLC). |
|
With ammonium sulfate;Heating / reflux; |
(A) A mixture of 5-Azacytosine (20 g, 178.4 mmol), ammonium sulfate (2.4 g, 18.16 mmol), and hexamethyldisilazane (160 g, 991.3 mmol) was heated to reflux until a clear solution was obtained. The excess of hexamethyldisilazane was removed in the vacuum at 60C. |
|
ammonium sulfate; at 120 - 134℃;Product distribution / selectivity; |
Hexamethyldisilazane (94.5 ml_), ammonium sulfate (1.25 g) and 5- azacytosine (25 g) were placed into a clean and dry round bottom flask and stirred. The mixture was heated to reflux at 120-1340C and maintained for 2 to 3 hours. The mixture was distilled under vacuum to give a residue and it was allowed to cool to 25-300C. Ethyl acetate (250 ml_) was charged to the residue and stirred. 1 ,2,3,5- tetra-O-acetyl-beta-D-ribofuranose (67.4 g) was added and the mixture was cooled to 5-10C. Triflic acid (23.6 ml_) was slowly added over 10-30 minutes and the mixture was allowed to warm to 20-300C and stirred for 1 -2 hours. Ethyl acetate (125 ml_) and water (125 ml_) were added and stirred. The organic layer was separated, washed with 20% sodium chloride solution (125 ml_), and dried over sodium sulfate. The organic layer was distilled under vacuum at about 40 to 45C to give a residue of 2,3,5-tri-O-acetyl-5-azacytidine (50.0 g). |
|
With ammonium sulphate; at 117℃; for 2h;Inert atmosphere; |
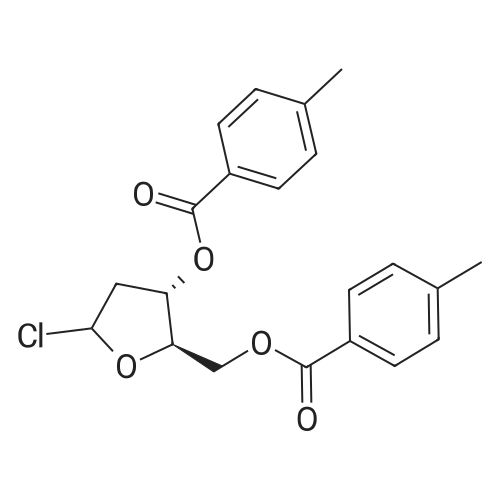
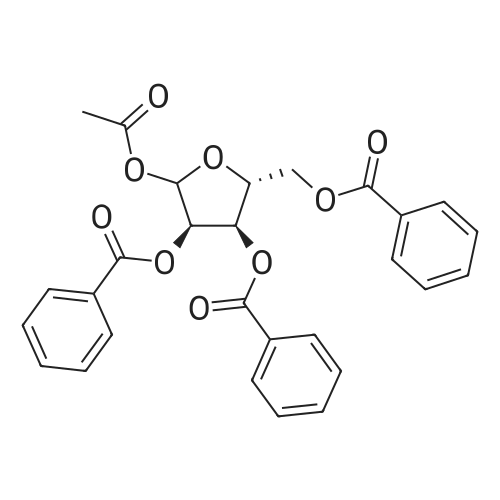
Example 6 Preparation of 4-amino-1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-alpha-D-ribofuranosyl-1,3,5-triazin-2(1H)-one and 4-amino-1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-beta-D-ribofuranosyl-1,3,5-triazin-2(1H)-one.Purified <strong>[931-86-2]5-azacytosine</strong> (1.62 g), ammonium sulfate (0.162 g) and hexamethyldisilazane (40 mL) were charged into a 4-neck, 100 mL round-bottom flask under nitrogen. The mixture was heated to 117 C. under stirring and kept at this temperature for 2 hours to give a clear solution. Unreacted hexamethyldisilazane was removed under vacuum to give 2-[(trimethylsilyl)amino]-4-[(trimethylsilyl)oxy]-s-triazine as a white solid. The solid was dissolved in anhydrous chloroform (25 mL) and was added into a solution of 1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-alpha-D-ribofuranosyl-chloride (5.0 g, from Example 2) in anhydrous chloroform and stirred at 1 C. under nitrogen. Trimethylsilyl trifluoromethanesulfonate (1.0 g) in anhydrous chloroform (10 mL) was added and the resulting mixture was stirred at this temperature for 21 hours. Saturated aqueous sodium bicarbonate was added to neutralize to pH 6. The mixture was diluted with additional chloroform (100 mL). After phase cut, the organic layer was washed with brine (30 mL) and dried over magnesium sulfate. The mixture was filtered through a bed of filter aid. Methanol was added and the solution was concentrated to 20 mL followed by the addition of anhydrous hexanes (40 mL) under stirring to form slurry. The solid was collected and dried under vacuum to give the product (5.06 g) that contains a mixture of 2.5:1 beta:alpha anomers. It is ready to be used in a process according to this invention for preparing Decitabine, without further purification. |
|
ammonium sulfate; at 125 - 130℃; for 6h;Inert atmosphere;Product distribution / selectivity; |
5-Azacytosine (200.0 g, 1.7842 mol, purity > 98%) and hexamethyldisilazane (HMDS) (1.4 L, 6.72 mol, purity > 98%) were charged into a 3-L 4-neck round bottom flask at 25-30C under nitrogen atmosphere. Ammonium sulfate (10.0 g, 0.0756 mol) was added. The mixture was gradually heated to reflux at 125-130C. The reflux was maintained for 6 hours. Typically, the reaction mass became a clear solution after 2-4 hours, and the reaction was substantially complete as soon as the clear solution was formed.[00271] The reaction mass was gradually cooled to 40-50C. HMDS was distilled off at 40-50C under vacuum (10-15 mmHg) to give a white solid. Nitrogen was used to break the vacuum over the solid. Toluene (400.0 mL) was added to the solid residue at 25-30C, and the solvent was distilled off at 40-50C under vacuum (10-15 mmHg) to yield a solid. Nitrogen was used to break the vacuum over the solid. The solid was gradually cooled to 25-30C, and carried through to the next step. HMDS was recovered in 75-80% yield with a purity of about 90-95%. |
| 7 g |
With ammonium sulfate; chloro-trimethyl-silane; for 17h;Reflux; |
(3) 5-Azacytosine (98%, Aldrich, 5.0 g, 44.8 mmol) and ammonium sulfate (25 mg) were suspended in hexamethyldisilazane (25 ml) and chlorotrimethylsilane (0.2 ml) was added. The reaction mixture was heated at the reflux for 17 h. The clear solution was cooled, evaporated, coevaporated twice with dry xylene and vacuum dried to yield whitish solids of the silylated 5-azacyto sine (.-7 g) which is used in whole for the next glycosylation step. |
|
With ammonium sulfate; for 4h;Reflux; Inert atmosphere; |
Under nitrogen atmosphere, a three-necked flask, followed by adding 500g5_ azacytosine, 12. 5g ammonium sulfate and 4000mlHMDS, the reaction was refluxed under stirring until the solution became clear and then refluxed for 4 hours, then evaporated under reduced pressure of HMDS, was pale yellow oil intermediate IV. |
| 0.521 kg |
With ammonium sulfate; at 120 - 140℃; for 18h; |
In 10L reaction flask was added <strong>[931-86-2]5-aza-cytosine</strong> (0.228kg, 2.04mol), ammonium sulfate powder (5.9g, 0.04mol) and HMDS (6.1L), was heated to maintain gentle reflux at 120-140 deg.] C, reaction was carried out for about 18 hr, the reaction was completed, the reaction was stopped, under stirring, under reduced pressure the excess HMDS was evaporated to give a yellow oil 0.521kg, i.e. intermediate 4 '. |
|
With ammonium sulfate; for 2h;Inert atmosphere; Reflux; |
Under nitrogen protection, 500g of <strong>[931-86-2]5-azacytosine</strong> was added to 3000ml of HMDS, and 5g of ammonium sulfate was added as a catalyst.The reaction was refluxed with stirring until the solution became clear, and the mixture was refluxed for 2 hours, then HMDS was evaporated to dryness under reduced pressure to afford Intermediate IV. |
| 19.5 g |
With ammonium sulfate; for 18h;Reflux; |
Mix <strong>[931-86-2]5-azacytosine</strong> (10.0 g), hexamethyldisilazane (200 mL) and ammonium sulfate (0.2 g), heat to reflux,The reaction was stirred for 18 hours. After the reaction is completed, it is concentrated under reduced pressure.Heat n-heptane (200 mL) to beat and filter.The filter cake was dried at 50 C for 12 hours.To give 19.5 g of white silyl <strong>[931-86-2]5-azacytosine</strong>,The mass yield was 195% (based on <strong>[931-86-2]5-azacytosine</strong>). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping