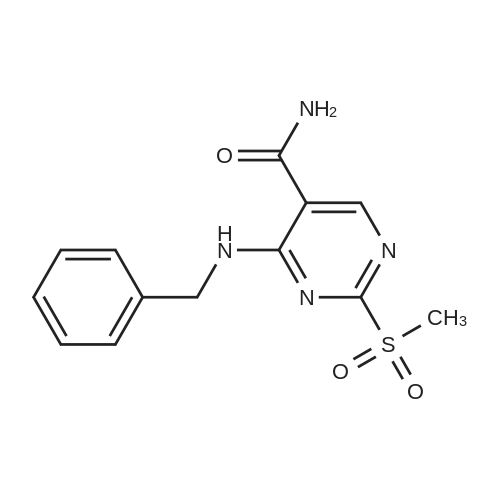
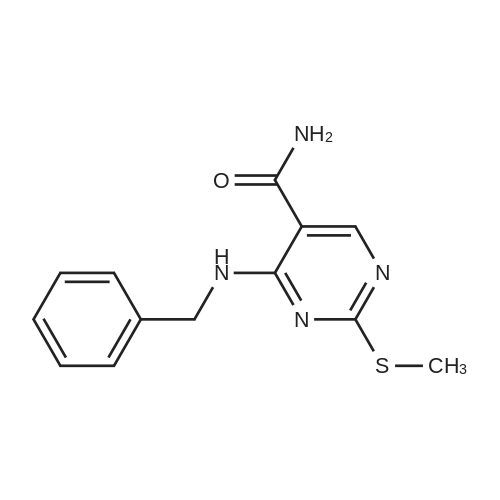
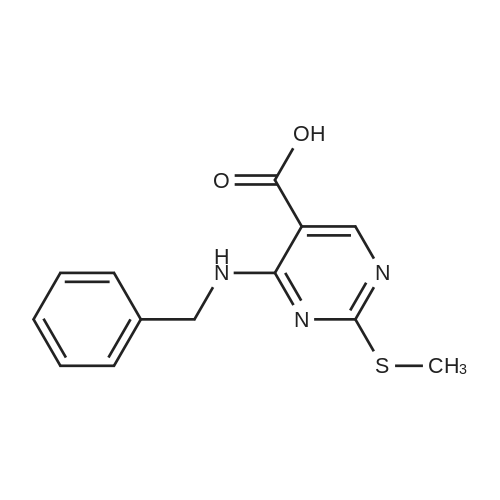
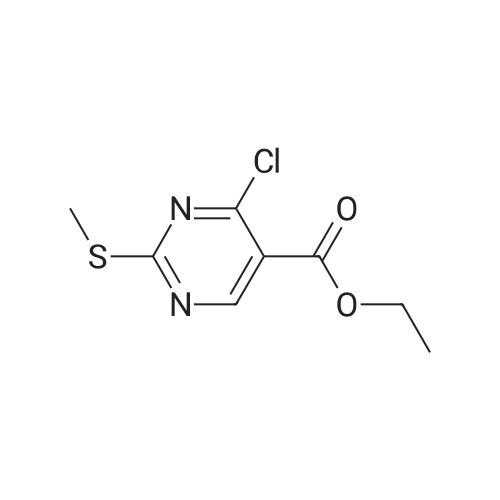
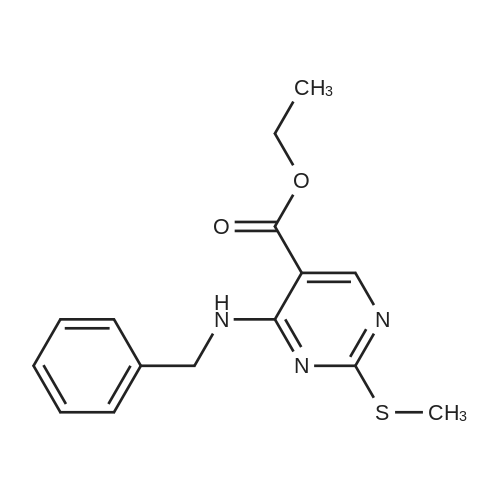
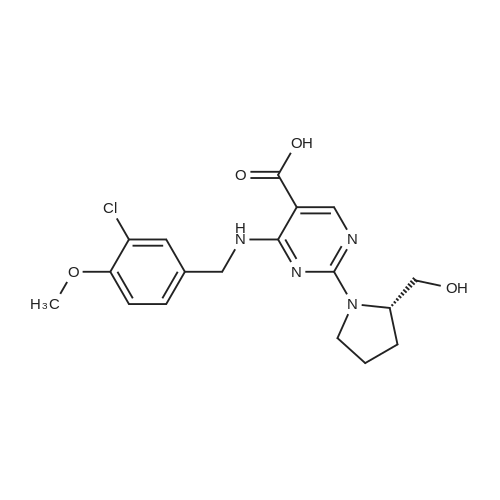
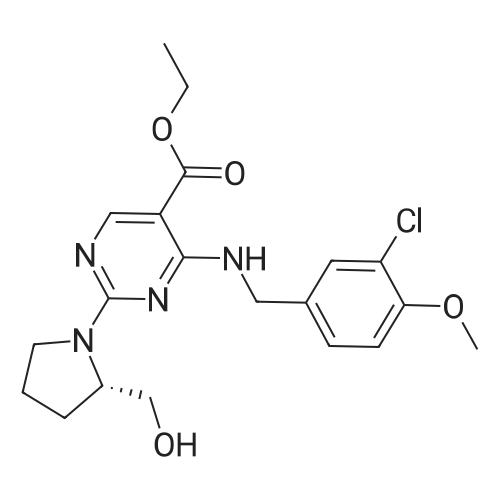
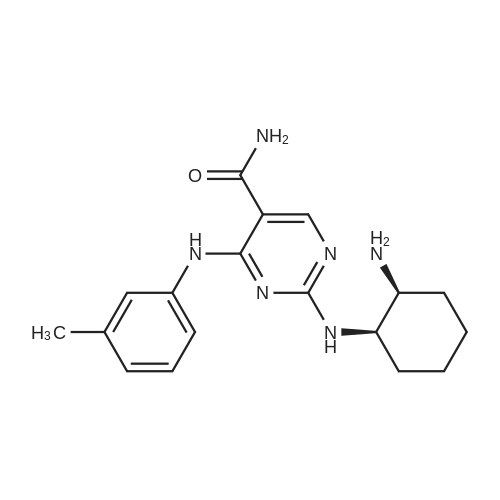
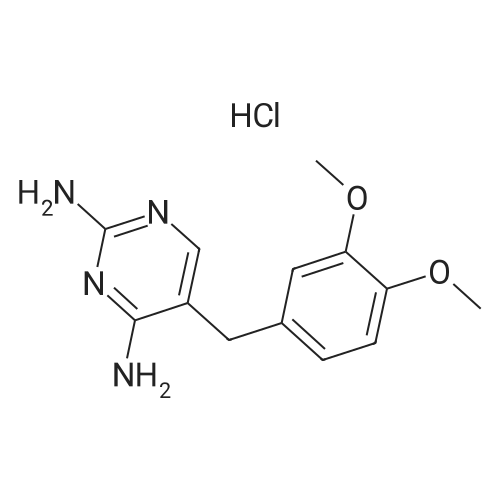
Alternatived Products of [ 919486-40-1 ]
Product Details of [ 919486-40-1 ]
CAS No. : 919486-40-1
MDL No. : MFCD24038757
Formula :
C20 H20 ClN5 O2
Boiling Point : -
Linear Structure Formula : -
InChI Key : OZRMEKAUZBKTTC-UHFFFAOYSA-N
M.W :
397.86
Pubchem ID : 10340781
Synonyms :
Chemical Name : 4-(Benzylamino)-2-((3-chloro-4-hydroxyphenethyl)amino)pyrimidine-5-carboxamide
Calculated chemistry of [ 919486-40-1 ]
Physicochemical Properties
Num. heavy atoms : 28
Num. arom. heavy atoms : 18
Fraction Csp3 : 0.15
Num. rotatable bonds : 8
Num. H-bond acceptors : 4.0
Num. H-bond donors : 4.0
Molar Refractivity : 109.55
TPSA : 113.16 Ų
Pharmacokinetics
GI absorption : High
BBB permeant : No
P-gp substrate : No
CYP1A2 inhibitor : Yes
CYP2C19 inhibitor : No
CYP2C9 inhibitor : Yes
CYP2D6 inhibitor : Yes
CYP3A4 inhibitor : Yes
Log Kp (skin permeation) : -5.89 cm/s
Lipophilicity
Log Po/w (iLOGP) : 2.91
Log Po/w (XLOGP3) : 3.99
Log Po/w (WLOGP) : 2.67
Log Po/w (MLOGP) : 2.17
Log Po/w (SILICOS-IT) : 2.87
Consensus Log Po/w : 2.92
Druglikeness
Lipinski : 0.0
Ghose : None
Veber : 0.0
Egan : 0.0
Muegge : 0.0
Bioavailability Score : 0.55
Water Solubility
Log S (ESOL) : -4.77
Solubility : 0.00679 mg/ml ; 0.0000171 mol/l
Class : Moderately soluble
Log S (Ali) : -6.07
Solubility : 0.00034 mg/ml ; 0.000000855 mol/l
Class : Poorly soluble
Log S (SILICOS-IT) : -7.56
Solubility : 0.000011 mg/ml ; 0.0000000276 mol/l
Class : Poorly soluble
Medicinal Chemistry
PAINS : 0.0 alert
Brenk : 0.0 alert
Leadlikeness : 3.0
Synthetic accessibility : 2.78
Safety of [ 919486-40-1 ]
Signal Word: Warning
Class: N/A
Precautionary Statements: P280-P305+P351+P338 UN#: N/A
Hazard Statements: H302 Packing Group: N/A
GHS Pictogram:
Application In Synthesis of [ 919486-40-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
Downstream synthetic route of [ 919486-40-1 ]
1
[ 643086-98-0 ]
[ 35607-19-3 ]
[ 919486-40-1 ]
Yield Reaction Conditions Operation in experiment
29%
With 1-methyl-pyrrolidin-2-one; diisopropylamine at 100℃; for 1h;
With N-ethyl-N,N-diisopropylamine In 1-methyl-pyrrolidin-2-one at 110℃; for 1h;
1
Example 1 To 8 ml NMP solution of 750 mg of 4-benzylamino-2-methylsulfonylpyrimidine-5-carboxamide were added 765 mg of 2-(3-chloro-4-hydroxyphenyl)ethylamine hydrochloride and 1.07 ml of diisopropylethylamine, followed by stirring at 110°C for 1 hour. The reaction mixture was cooled down to room temperature, and then mixed with water and extracted with ethyl acetate. The organic layer was washed with saturated brine, and then the solvent was evaporated. The resulting residue was purified by a silica gel column chromatography (chloroform:methanol:aqueous ammonia) and the resulting crude crystals were recrystallized (methanol-ethyl acetate) to obtain 280 mg of 4-benzylamino-2-[2-(3-chloro-4-hydroxyphenyl)ethyl]amino}pyrimidine-5-carboxamide as colorless crystals.
Reference:
[1]Nagashima, Shinya; Yokota, Masaki; Nakai, Ei-ichi; Kuromitsu, Sadao; Ohga, Keiko; Takeuchi, Makoto; Tsukamoto, Shin-ichi; Ohta, Mitsuaki
[Bioorganic and Medicinal Chemistry, 2007, vol. 15, # 2, p. 1044 - 1055]
[2]Current Patent Assignee: ASTELLAS PHARMA INC - EP1518855, 2005, A1
Location in patent: Page/Page column 23
2
[ 919486-26-3 ]
[ 919486-40-1 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 2 steps
1: 91 percent / N-methylpyrrolidinone; m-chloroperbenzoic acid / 3 h
2: 29 percent / diisopropylamine; N-methylpyrrolidinone / 1 h / 100 °C
Reference:
[1]Nagashima, Shinya; Yokota, Masaki; Nakai, Ei-ichi; Kuromitsu, Sadao; Ohga, Keiko; Takeuchi, Makoto; Tsukamoto, Shin-ichi; Ohta, Mitsuaki
[Bioorganic and Medicinal Chemistry, 2007, vol. 15, # 2, p. 1044 - 1055]
3
[ 686267-34-5 ]
[ 919486-40-1 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 3 steps
1: 98 percent / 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; 1-hydroxybenzotriazole; NH4 OH / dimethylformamide; H2 O / 16 h / 20 °C
2: 91 percent / N-methylpyrrolidinone; m-chloroperbenzoic acid / 3 h
3: 29 percent / diisopropylamine; N-methylpyrrolidinone / 1 h / 100 °C
Reference:
[1]Nagashima, Shinya; Yokota, Masaki; Nakai, Ei-ichi; Kuromitsu, Sadao; Ohga, Keiko; Takeuchi, Makoto; Tsukamoto, Shin-ichi; Ohta, Mitsuaki
[Bioorganic and Medicinal Chemistry, 2007, vol. 15, # 2, p. 1044 - 1055]
4
[ 5909-24-0 ]
[ 919486-40-1 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 5 steps
1: 96 percent / ethyldiisopropylamine / acetonitrile / 5 h / 20 °C
2: 100 percent / NaOH / methanol; tetrahydrofuran / 5 h / 50 °C
3: 98 percent / 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; 1-hydroxybenzotriazole; NH4 OH / dimethylformamide; H2 O / 16 h / 20 °C
4: 91 percent / N-methylpyrrolidinone; m-chloroperbenzoic acid / 3 h
5: 29 percent / diisopropylamine; N-methylpyrrolidinone / 1 h / 100 °C
Reference:
[1]Nagashima, Shinya; Yokota, Masaki; Nakai, Ei-ichi; Kuromitsu, Sadao; Ohga, Keiko; Takeuchi, Makoto; Tsukamoto, Shin-ichi; Ohta, Mitsuaki
[Bioorganic and Medicinal Chemistry, 2007, vol. 15, # 2, p. 1044 - 1055]
5
[ 100973-67-9 ]
[ 919486-40-1 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 4 steps
1: 100 percent / NaOH / methanol; tetrahydrofuran / 5 h / 50 °C
2: 98 percent / 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; 1-hydroxybenzotriazole; NH4 OH / dimethylformamide; H2 O / 16 h / 20 °C
3: 91 percent / N-methylpyrrolidinone; m-chloroperbenzoic acid / 3 h
4: 29 percent / diisopropylamine; N-methylpyrrolidinone / 1 h / 100 °C
Reference:
[1]Nagashima, Shinya; Yokota, Masaki; Nakai, Ei-ichi; Kuromitsu, Sadao; Ohga, Keiko; Takeuchi, Makoto; Tsukamoto, Shin-ichi; Ohta, Mitsuaki
[Bioorganic and Medicinal Chemistry, 2007, vol. 15, # 2, p. 1044 - 1055]
6
[ 100-46-9 ]
[ 919486-40-1 ]
Yield Reaction Conditions Operation in experiment
Multi-step reaction with 5 steps
1: 96 percent / ethyldiisopropylamine / acetonitrile / 5 h / 20 °C
2: 100 percent / NaOH / methanol; tetrahydrofuran / 5 h / 50 °C
3: 98 percent / 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; 1-hydroxybenzotriazole; NH4 OH / dimethylformamide; H2 O / 16 h / 20 °C
4: 91 percent / N-methylpyrrolidinone; m-chloroperbenzoic acid / 3 h
5: 29 percent / diisopropylamine; N-methylpyrrolidinone / 1 h / 100 °C
Reference:
[1]Nagashima, Shinya; Yokota, Masaki; Nakai, Ei-ichi; Kuromitsu, Sadao; Ohga, Keiko; Takeuchi, Makoto; Tsukamoto, Shin-ichi; Ohta, Mitsuaki
[Bioorganic and Medicinal Chemistry, 2007, vol. 15, # 2, p. 1044 - 1055]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping