| 29% |
|
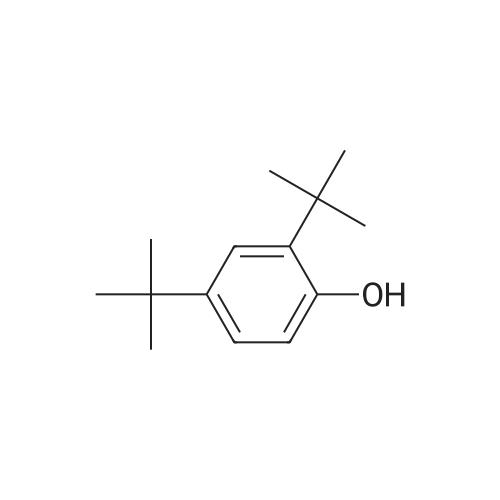
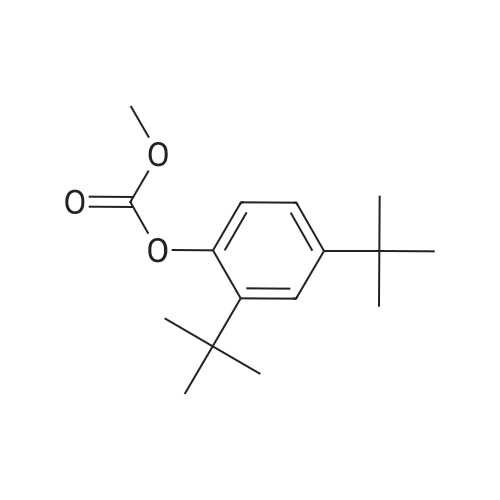
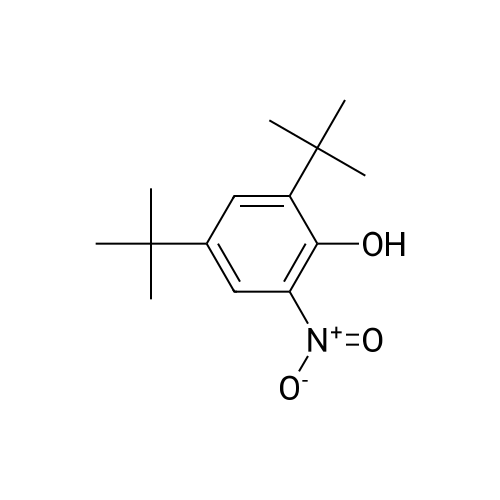
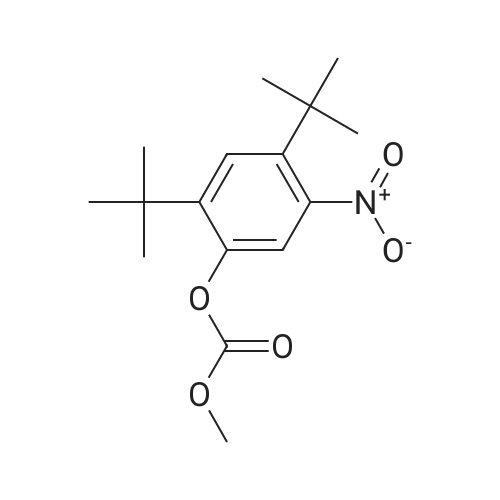
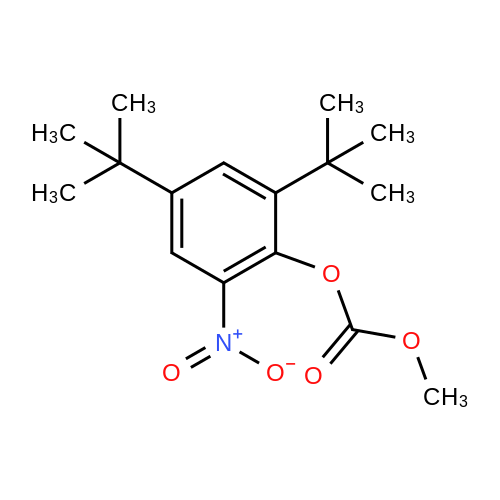
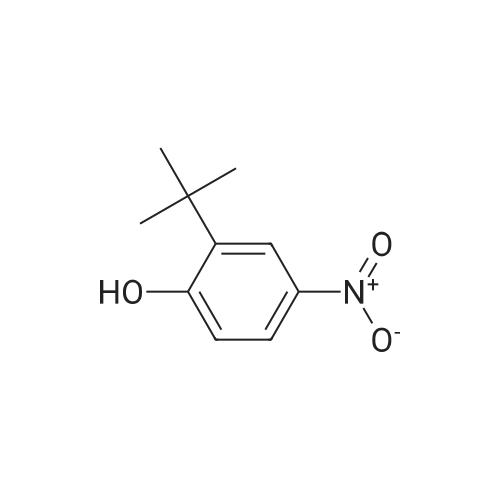
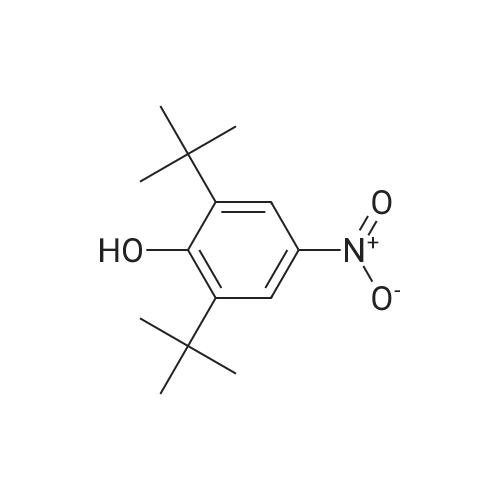
Carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and Carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester To a stirring mixture of carbonic acid 2,4-di-ie/ -butyl-phenyl ester methyl ester (4.76 g, 180 mmol) in cone, sulfuric acid (2 mL), cooled in an ice-water bath, was added a cooled mixture of sulfuric acid (2 mL) and nitric acid (2 mL). The addition was done slowly so that the reaction temperature did not exceed 50 C. The reaction was allowed to stir for 2 h while warming to room temperature. The reaction mixture was then added to ice-water and extracted into diethyl ether. The ether layer was dried (MgS04), concentrated and purified by column chromatography (0 - 10% ethyl acetate - hexanes) to yield a mixture of carbonic acid 2,4-di-ieri-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-ieri-butyl-6-nitro-phenyl ester methyl ester as a pale yellow solid (4.28 g), which was used directly in the next step. Step 3: 2,4-Di-tert-butyl-5-nitro-phenol and 2,4-Di-tert-butyl-6-nitro-phenol (1128) [00214] The mixture of carbonic acid 2,4-di-ieri-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-ieri-butyl-6-nitro-phenyl ester methyl ester (4.2 g, 14.0 mmol) was dissolved in MeOH (65 mL) before KOH (2.0 g, 36 mmol) was added. The mixture was stirred at room temperature for 2 h. The reaction mixture was then made acidic (pH 2-3) by adding cone. HCl and partitioned between water and diethyl ether. The ether layer was dried (MgS04), concentrated and purified by column chromatography (0 - 5 % ethyl acetate - hexanes) to provide 2,4-di-ieri-butyl-5-nitro-phenol (1.31 g, 29% over 2 steps) and 2,4-di-ieri-butyl-6-nitro-phenol. 2,4-Di-ieri-butyl-5-nitro-phenol: XH NMR (400 MHz, DMSO-ifc) delta 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, 9H). 2,4-Di-ieri-butyl-6-nitro-phenol: lH NMR (400 MHz, CDC13) delta 11.48 (s, 1H), 7.98 (d, J = 2.5 Hz, 1H), 7.66 (d, J = 2.4 Hz, 1H), 1.47 (s, 9H), 1.34 (s, 9H). |
| 29% |
|
To a stirring mixture of carbonic acid 2,4-di-ie/ -butyl-phenyl ester methyl ester (4.76 g, 180 mmol) in cone sulfuric acid (2 mL), cooled in an ice-water bath, was added a cooled mixture of sulfuric acid (2 mL) and nitric acid (2 mL). The addition was done slowly so that the reaction temperature did not exceed 50 C. The reaction was allowed to stir for 2 h while warming to room temperature. The reaction mixture was then added to ice-water and extracted into diethyl ether. The ether layer was dried (MgS04), concentrated and purified by column chromatography (0 - 10% ethyl acetate - hexanes) to yield a mixture of carbonic acid 2,4-di-ie/ -butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-/er/-butyl-6-nitro-phenyl ester methyl ester as a pale yellow solid (4.28 g), which was used directly in the next step.Step 3: 2,4-Di-tert-butyl-5-nitrophenol and 2,4-Di-tert-butyl-6-nitrophenol [00429] The mixture of carbonic acid 2,4-di-tert-butyl-5-nitrophenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitrophenyl ester methyl ester (4.2 g, 14.0 mmol) was dissolved in MeOH (65 mL) before KOH (2.0 g, 36 mmol) was added. The mixture was stirred at room temperature for 2 h. The reaction mixture was then made acidic (pH 2-3) by adding cone. HC1 and partitioned between water and diethyl ether. The ether layer was dried (MgS04), concentrated and purified by column chromatography (0 - 5 % ethyl acetate - hexanes) to provide 2,4-di-tert-butyl-5-nitrophenol (1.31 g, 29% over 2 steps) and 2,4-di-tert-butyl-6-nitrophenol. 2,4-Di-tert-butyl-5-nitrophenol : 1H NMR (400 MHz, DMSO-rfc) d 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, 9H). 2,4-Di-tert-butyl-6-nitrophenol: 1H NMR (400 MHz, CDCb) d 11.48 (s, 1H), 7.98 (d, J = 2.5 Hz, 1H), 7.66 (d, J = 2.4 Hz, 1H), 1.47 (s, 9H), 1.34 (s, 9H). |
| 29% |
|
To a stirring mixture of carbonic acid 2,4-di-tert-butyl-phenyl ester methyl ester (4.76 g, 180 mmol) in conc. sulfuric acid (2 mL), cooled in an ice-water bath, was added a cooled mixture of sulfuric acid (2 mL) and nitric acid (2 mL). The addition was done slowly so that the reaction temperature did not exceed 50 C. The reaction was allowed to stir for 2 h while warming to room temperature. The reaction mixture was then added to ice-water and extracted into diethyl ether. The ether layer was dried (MgSO4), concentrated and purified by column chromatography (0-10% ethyl acetate-hexanes) to yield a mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester as a pale yellow solid (4.28 g), which was used directly in the next step. The mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester (4.2 g, 14.0 mmol) was dissolved in MeOH (65 mL) before KOH (2.0 g, 36 mmol) was added. The mixture was stirred at room temperature for 2 h. The reaction mixture was then made acidic (pH 2-3) by adding conc. HCl and partitioned between water and diethyl ether. The ether layer was dried (MgSO4), concentrated and purified by column chromatography (0-5% ethyl acetate-hexanes) to provide 2,4-di-tert-butyl-5-nitro-phenol (1.31 g, 29% over 2 steps) and 2,4-di-tert-butyl-6-nitro-phenol. 2,4-Di-tert-butyl-5-nitro-phenol: 1H NMR (400 MHz, DMSO-d6) delta 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, 9H). 2,4-Di-tert-butyl-6-nitro-phenol: 1H NMR (400 MHz, CDCl3) delta 11.48 (s, 1H), 7.98 (d, J=2.5 Hz, 1H), 7.66 (d, J=2.4 Hz, 1H), 1.47 (s, 9H), 1.34 (s, 9H). |
| 29% |
|
The mixture of carbonic acid 2,4-di-fer/-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-/er/-butyl-6-nitro-phenyl ester methyl ester (4.2 g, 14.0 mmol) was dissolved in MeOH (65 mL) before KOH (2.0 g, 36 mmol) was added. The mixture was stirred at room temperature for 2 h. The reaction mixture was then made acidic (pH 2-3) by adding cone. HC1 and partitioned between water and diethyl ether. The ether layer was dried (MgSCri), concentrated and purified by column chromatography (0 - 5 % ethyl acetate - hexanes) to provide 2,4-di-/er/-butyl-5-nitro-phenol (1.31 g, 29% over 2 steps) and 2,4-di-/c/V-butyl-6-nitro-phenol. 2,4-Di-/er/-butyl-5-nitro-phenol: 1H NMR (400 MHz, DMSO-i) d 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, (0431) 9H). 2,4-Di-/er/-butyl-6-nitro-phenol: NMR (400 MHz, CDCb) d 11.48 (s, 1H), 7.98 (d, J = 2.5 Hz, 1H), 7.66 (d, J = 2.4 Hz, 1H), 1.47 (s, 9H), 1.34 (s, 9H). |
|
|
The mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester (4.2 g, 12.9 mmol) was dissolved in MeOH (65 mL) and KOH (2.0 g, 36 mmol) was added. The mixture was stirred at room temperature for 2 h. The reaction mixture was then made acidic (pH 2-3) by adding conc. HCl and partitioned between water and diethyl ether. The ether layer was dried (MgSO4), concentrated and purified by column chromatography (0-5% ethyl acetate-hexanes) to provide 2,4-di-tert-butyl-5-nitro-phenol (1.31 g, 29% over 2 steps) and 2,4-di-tert-butyl-6-nitro-phenol. 2,4-Di-tent-butyl-5-nitro-phenol: 1H NMR (400 MHz, DMSO-d6) delta 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, 9H). 2,4-Di-tert-butyl-6-nitro-phenol: 1H NMR (400 MHz, CDCl3) delta 11.48 (s, 1H), 7.98 (d, J=2.5 Hz, 1H), 7.66 (d, J=2.4 Hz, 1H), 1.47 (s, 9H), 1.34 (s, 9H) |
|
|
To a stirring mixture of carbonic acid 2,4-di-tert-butyl-phenyl ester methyl ester (4.76 g, 180 mmol) in cone, sulfuric acid (2 mL), cooled in an ice-water bath, was added a cooled mixture of sulfuric acid (2 mL) and nitric acid (2 mL). The addition was done slowly so that the reaction temperature did not exceed 50 C. The reaction was allowed to stir for 2 h while warming to room temperature. The reaction mixture was then added to ice-water and extracted into diethyl ether. The ether layer was dried (MgSO4) concentrated and purified by column chromatography (0 - 10% ethyl acetate - hexanes) to yield a mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester as a pale yellow solid (4.28 g), which was used directly in the next step. The mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester (4.2 g, 14.0 mmol) was dissolved in MeOH (65 mL) before KOH (2.0 g, 36 mmol) was added. The mixture was stirred at room temperature for 2 h. The reaction mixture was then made acidic (pH 2-3) by adding cone. HC1 and partitioned between water and diethyl ether. The ether layer was dried (MgSO4), concentrated and purified by column chromatography (0 - 5 % ethyl acetate - hexanes) to provide 2,4-di-tert-butyl-5-nitro-phenol (1.31 g, 29% over 2 steps) and 2,4-di-tert-butyl-6-nitro-phenol. 2,4-Oi-tert-butyl-5-nitro-phenol: 1H NMR (400 MHz, DMSO-d6) delta 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, 9H). 2,4-Di-tert-butyl-6-nitro-phenol: 1H NMR (400 MHz, CDCl3) delta 11.48 (s, 1H), 7.98 (d, J = 2.5 Hz, 1H), 7.66 (d, J = 2.4 Hz, 1H), 1.47 (s, 9H), 1.34 (s, 9H). |
|
|
To a stirring mixture of carbonic acid 2,4-di-tert-butyl-phenyl ester methyl ester (4.76 g, 180 mmol) in conc. sulfuric acid (2 mL), cooled in an ice-water bath, was added a cooled mixture of sulfuric acid (2 mL) and nitric acid (2 mL). The addition was done slowly so that the reaction temperature did not exceed 50 ^C. The reaction was allowed to stir for 2 h while warming to room temperature. The reaction mixture was then added to ice-water and extracted into diethyl ether. The ether layer was dried (MgSO4), concentrated and purified by column chromatography (0- 10% ethyl acetate - hexanes) to yield a mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester as a pale yellow solid (4.28 g), which was used directly in the next step. The mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester (4.2 g, 14.0 mmol) was dissolved in MeOH (65 mL) before KOH (2.0 g, 36 mmol) was added. The mixture was stirred at room temperature for 2 h. The reaction mixture was then made acidic (pH 2-3) by adding conc. HCl and partitioned between water and diethyl ether. The ether layer was dried (MgSO4), concentrated and purified by columnchromatography (0- 5 % ethyl acetate- hexanes) to provide 2,4-di-tert-butyl-5-nitro- phenol (1.31 g, 29% over 2 steps) and 2,4-di-tert-butyl-6-nitro-phenol. 2,4-Di-tert- butyl-5-nitro-phenol:1H NMR (400 MHz, DMSO-d6) delta 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, 9H). 2,4-Di-tert-butyl-6-nitro-phenol:1H NMR (400 MHz, CDCl3) delta 11.48 (s, 1H), 7.98 (d, J = 2.5 Hz, 1H), 7.66 (d, J = 2.4 Hz, 1H), 1.47 (s, 9H), 1.34 (s, 9H). |
| 1.31 g |
|
To a stirring mixture of carbonic acid 2,4-di-tert-butyl-phenyl ester methyl ester (4.76 g, 180 mmol) in conc. sulfuric acid (2 mL), cooled in an ice-water bath, was added a cooled mixture of sulfuric acid (2 mL) and nitric acid (2 mL). The addition was done slowly so that the reaction temperature did not exceed 50 C. The reaction was allowed to stir for 2 h while warming to room temperature. The reaction mixture was then added to ice-water and extracted into diethyl ether. The ether layer was dried (MgSO4), concentrated and purified by column chromatography (0-10% ethyl acetate hexanes) to yield a mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester as a pale yellow solid (4.28 g), which was used directly in the next step. Step C: 2,4-Di-tert-butyl-5-nitro-phenol and 2,4-Di-tert-butyl-6-nitro-phenol (0101) The mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester (4.2 g, 14.0 mmol) was dissolved in 50 MeOH (65 mL) before 51 KOH (2.0 g, 36 mmol) was added. The mixture was stirred at room temperature for 2 h. The reaction mixture was then made acidic (pH 2-3) by adding cone. HCl and partitioned between water and diethyl ether. The ether layer was dried (MgSO4), concentrated and purified by column chromatography (0-5% ethyl acetate-hexanes) to provide 52 2,4-di-tert-butyl-5-nitro-phenol (1.31 g, 29% over 2 steps) and 2,4-di-tert-butyl-6-nitro-phenol. 2,4-Di-tert-butyl-5-nitro-phenol: 1H NMR (400 MHz, DMSO-d6) delta 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, 9H) ppm. 2,4-Di-tert-butyl-6-nitro-phenol: 1H NMR (400 MHz, CDCl3) delta 11.48 (s, 1H), 7.98 (d, J=2.5 Hz, 1H), 7.66 (d, J=2.4 Hz, 1H), 1.47 (s, 9H), 1.34 (s, 9H) ppm. |
| 1.31 g |
|
1003371 To a stirring mixture of carbonic acid 2,4-di-tert-butyl-phenyl ester methyl ester (4.76 g, 180 mmol) in conc. sulfuric acid (2 mL), cooled in an ice-water bath, was added a cooled mixture of sulfuric acid (2 mL) and nitric acid (2 mL). The addition was done slowly so that the reaction temperature did not exceed 50 C. The reaction was allowed to stir for 2 h while warming to room temperature. The reaction mixture was then added to ice-water and extracted into diethyl ether. The ether layer was dried (MgSO4), concentrated and purified by column chromatography (0 - 10% ethyl acetate - hexanes) to yield a mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and Ccarbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester as a pale yellow solid (4.28 g), which was used directly in the next step.; The mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester (4.2 g, 14.0 mmol) was dissolved in MeOH (65 mL) before KOH (2.0 g, 36 mmol) was added. The mixture was stirred at room temperature for 2 h. The reaction mixture was then made acidic (pH 2-3) by adding conc. HC1 and partitioned between water and diethyl ether. The ether layer was dried (MgSO4), concentrated and purified by column chromatography (0 - 5 % ethyl acetate - hexanes) to provide 2,4-di-tert-butyl-5-nitro-phenol (1.31 g, 29% over 2 steps) and 2,4-di-tert-butyl-6-nitro-phenol. 2,4-Di-tert-butyl-5 -nitro-phenol: ?H NMR (400 IVIHz, DMSO-d6) 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, 9H). 2,4-Di-tert-butyl-6-nitro-phenol: ?H NMR (400 IVIFIz, CDC13) 11.48 (s, 1H), 7.98 (d, J = 2.5 Hz, 1H), 7.66 (d, J = 2.4 Hz, 1H), 1.47 (s, 9H), 1.34 (s, 9H). |
| 1.31 g |
|
To a stirring mixture of carbonic acid 2,4-di -//77-butyl -phenyl ester methyl ester (4.76 g, 180 mmol) in cone sulfuric acid (2 mL), cooled in an ice-water bath, was added a cooled mixture of sulfuric acid (2 mL) and nitric acid (2 mL). The addition was done slowly so that the reaction temperature did not exceed 50 C. The reaction was allowed to stir for 2 h while warming to room temperature. The reaction mixture was then added to ice-water and extracted into diethyl ether. The ether layer was dried (MgS04), concentrated and purified by column chromatography (0 - 10% ethyl acetate - hexanes) to yield a mixture of carbonic acid 2,4-di-/er/-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-/er/-butyl-6-nitro-phenyl ester methyl ester as a pale yellow solid (4.28 g), which was used directly in the next step.; The mixture of carbonic acid 2,4-di-ie/t-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-/er/-butyl-6-nitro-phenyl ester methyl ester (4.2 g, 14.0 mmol) was dissolved in MeOH (65 mL) before KOH (2.0 g, 36 mmol) was added. The mixture was stirred at room temperature for 2 h. The reaction mixture was then made acidic (pH 2-3) by adding cone. HC1 and partitioned between water and diethyl ether. (0821) The ether layer was dried (MgS04), concentrated and purified by column (0822) chromatography (0 - 5 % ethyl acetate - hexanes) to provide 2, 4-di -//77-butyl -5-nitro- phenol (1.31 g, 29% over 2 steps) and 2,4-di-ie/7-butyl-6-nitro-phenol. 2,4-Di-ie/T-butyl- 5-nitro-phenol: 1H NMR (400 MHz, DMSO-zfc) d 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, 9H). 2,4-Di-ferf-butyl-6-nitro-phenol: 1H NMR (400 MHz, CDCb) d 11.48 (s, 1H), 7.98 (d, J = 2.5 Hz, 1H), 7.66 (d, J = 2.4 Hz, 1H), 1.47 (s, 9H), 1.34 (s, 9H). |
|
|
To a stirring mixture of carbonic acid 2,4-di-tert- butyl-phenyl ester methyl ester (4.76 g, 180 mmol) in conc. sulfuric acid (2 mE), cooled in an ice-water bath, was added a cooled mixture of sulfuric acid (2 mE) and nitric acid (2 mE). The addition was done slowly so that the reaction temperature did not exceed 50 C. The reaction was allowed to stir for 2 h while warming to room temperature. The reaction mixture was then added to ice-water and extracted into diethyl ether. The ether layer was dried (MgSO4), concentrated and purified by column chromatography (0-10% ethyl acetate-hexanes) to yield a mixture of carbonic acid 2,4-di-tert-butyl-5-nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert-butyl-6-nitro-phenyl ester methyl ester as a pale yellow solid (4.28 g), which was used directly in the next step.; The mixture of carbonic acid 2,4-di-tert-butyl-5- nitro-phenyl ester methyl ester and carbonic acid 2,4-di-tert- butyl-6-nitro-phenyl ester methyl ester (4.2 g, 14.0 mmol) was dissolved in MeOH (65 mE) before KOH (2.0 g, 36 mmol) was added. The mixture was stirred at room temperature for 2 h. The reaction mixture was then made acidic (pH 2-3) by adding conc. HC1 and partitioned between water and diethyl ethet The ether layer was dried (MgSO4), concentrated and purified by column chromatography (0-5% ethyl acetate-hexanes) to provide 2,4-di-tert-butyl-5-nitro- phenol (1.31 g, 29% over 2 steps) and 2,4-di-tert-butyl-6- nitro-phenol. 2,4-Di-tert-butyl-5-nitro-phenol: ?H NMR (400 MHz, DMSO-d6) oe 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, 9H). 2,4-Di-tert-butyl-6- nitro-phenol: ?H NMR (400 MHz, CDC13) oe 11.48 (s, 1H), 7.98 (d, J=2.5 Hz, 1H), 7.66 (d, J=2.4 Hz, 1H), 1.47 (s, 9H),1.34 (s, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping