Alternatived Products of [ 845872-49-3 ]
Product Details of [ 845872-49-3 ]
| CAS No. : | 845872-49-3 |
MDL No. : | MFCD06740319 |
| Formula : |
C8H7BO2S
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | LVRZWFSXTOTWTH-UHFFFAOYSA-N |
| M.W : |
178.02
|
Pubchem ID : | 16640610 |
| Synonyms : |
|
Safety of [ 845872-49-3 ]
Application In Synthesis of [ 845872-49-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 845872-49-3 ]
- 1
-
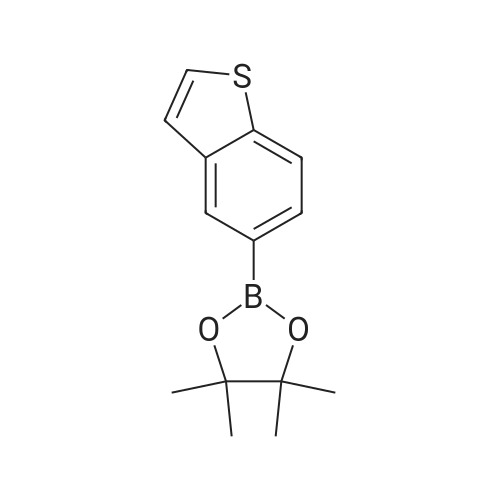 [ 501945-71-7 ]
[ 501945-71-7 ]

-
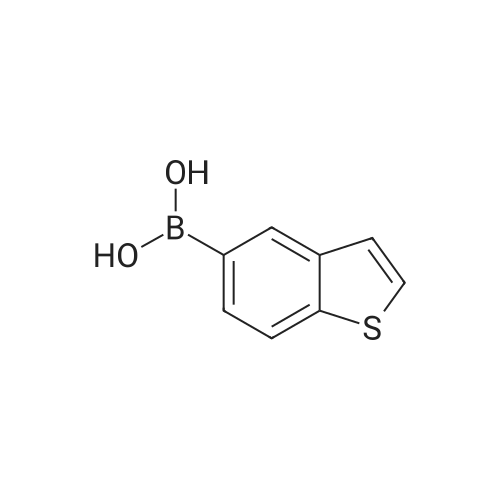 [ 845872-49-3 ]
[ 845872-49-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With boron trichloride; In dichloromethane; at -78℃; for 0.25h; |
A solution of 0.6 g of <strong>[501945-71-7]2-(1-benzothiophen-5-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane</strong> in 2.5 ml of dichloromethane is added dropwise to a solution of boron trichloride in dichloromethane (1 M, 14.3 ml) at -78C. After 15 minutes, the reaction mixture is warmed to room temperature, and 8 ml of methanol are added dropwise. It is then evaporated to dryness and dried under high vacuum overnight. 0.350 g of the title compound is obtained as a grey powder. Rt = 3.33. |
|
|
A solution of 0.6 g of 2-(l-benzothiophen-5-yl) 4,4,5,5-tetramethyl-1,3,2-dioxaborolane in 2.5 m of dichloromethane is added dropwise to a solution of boron trichloride in dichloromethane (1M, 14.3 ml) at -78 C. After 15 minutes, the reaction mixture is warmed to room temperature, and 8 ml of methanol are added dropwise. It is then evaporated to dryness and dried under high vacuum overnight. 0.350 g of the title compound is obtained as a grey powder Rt=3.33. |
|
|
A solution of 0.6 g of 2-(1-benzothiophen-5-yl)-4,4,5,5-tetramethyl-1 ,3,2-dioxaborolane in 2.5 ml of dichloromethane is added dropwise to a solution of boron trichloride in dichloromethane (1M, 14.3 ml) at -78C. After 15 minutes, the reaction mixture is warmed to room temperature, and 8 ml of methanol are added dropwise. It is then evaporated to dryness and dried under high vacuum overnight. 0.350 g of the title compound is obtained as a grey powder. Rt = 3.33. |
- 2
-
 [ 67-56-1 ]
[ 67-56-1 ]

-
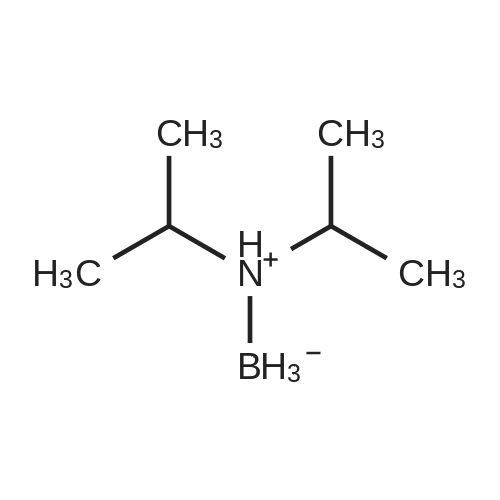
 [ 4923-87-9 ]
[ 4923-87-9 ]

-
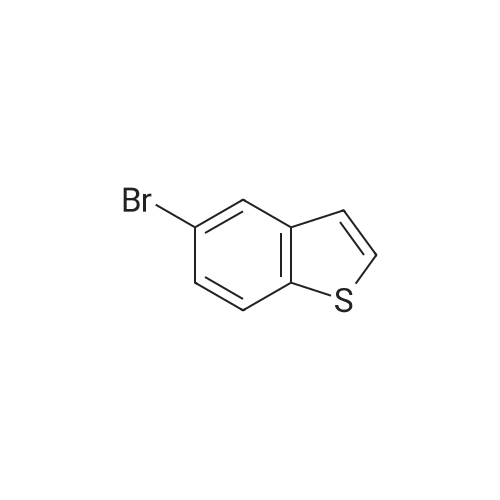
 [ 55124-35-1 ]
[ 55124-35-1 ]

-
 [ 845872-49-3 ]
[ 845872-49-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 93% |
|
General procedure: To a solution in THF (4 mL) of DIPAB (863 mg, 7.5 mmol) and Mg (182 mg, 7.5 mmol) were added a PhMgBr 1M THF solution (375 muL, 375mumol) at room temperature. After 10 min, 30 mL of anhydrous THF were added followed by the arylbromide (5 mmol). The reaction mixture was cooled down to 0 C and quenched slowly with 7 mL of MeOH. After 1h, volatile were removed under reduced pressure and the resulting solid was dissolved in 1N HCl/MeOH (7/3). After 1h at room temperature, 100 mL of AcOEt were added, the organic phase was washed with 1N HCl (30 mL) and brine (3×30 mL). Organic phases were concentrated under reduced pressure yielding a solid which was recrystallized from H2O. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping








