| 84.7% |
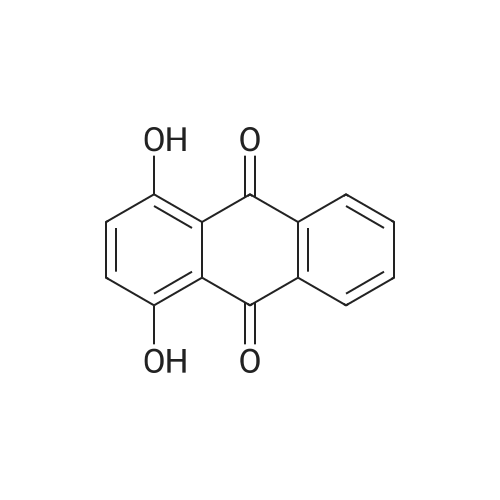
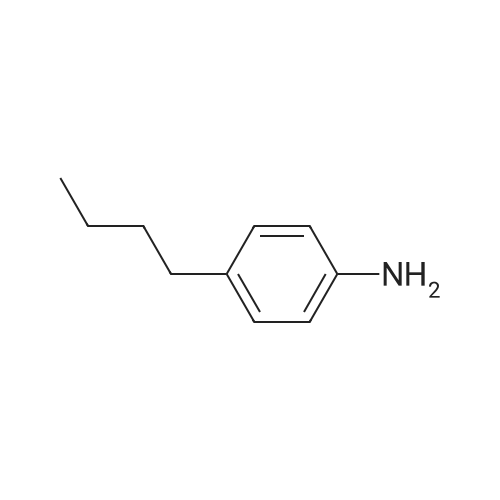
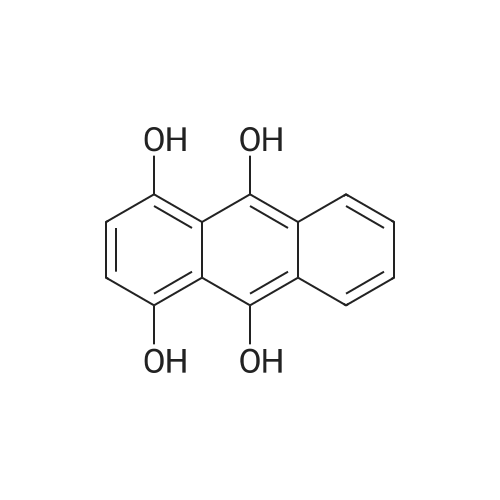
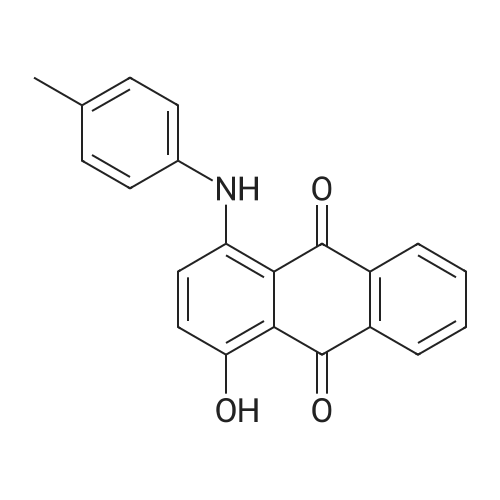
Stage #1: 1,4-dihydroxy-9,10-anthracenedione; <i>p</i>-toluidine With hydrogenchloride; benzoic acid; zinc In methanol; water at 60 - 116℃; for 19h;
Stage #2: With oxygen In methanol; water at 60℃; |
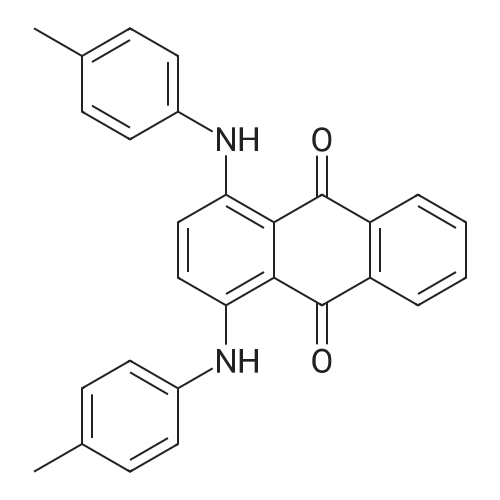
In a 500 mL pressure vessel equipped with a mechanical stirrer and a thermometer, 40 g of soft water was added,Hydrochloric acid 15 g,, 40 g of 4-dihydroxyanthraquinone, 25 g of p-toluidine (purchased), 160 ml of methanol, 11 g of benzoic acid,6 g of polyethylene glycol,Slowly heated to 60 ° C, the slow addition of zinc powder 5g,The temperature at 70 ° C for 2 hours, and then heated to 90 ° C for 8 hours, then continue to constant temperature to 110 ° C for 6 hours,Continue heating to 116 ° C for 3 hours.After completion of the reaction, the temperature was lowered to 60 ° C. After passing the air, the intermediate was rapidly oxidized and filtered. The filter cake was washed successively with methanol and hot water, and 50 g of solvent violet 13 was obtained. The purity was 93%, the yield was 84.7%, DC was -0.5, DH was -0.3 and the intensity was 99.50 |
|
With boric acid; sodium hydroxide In water at 115℃; Autoclave; |
1-3 Example 1:
Put 120ml water, 10g solid sodium hydroxide, 30g 1,4-dihydroxyanthraquinone, 4.6g 1,4-dihydroxyanthraquinone leuco in a 500ml autoclave, 7.2g p-toluidine, 2g boric acid, 0.5g of phase transfer catalyst, after stirring for 0.5h, the temperature is slowly raised to 115°C after sealing, after 3h, sampling is followed for detection, and the temperature is stopped after being kept for 6.5h. At this time, the content of 1,4-di-p-tolueneaminoanthraquinone is 0.67%, and the main content of the product is 53.8%. Cool down to 80, release the pressure, filter at this temperature, Then add 20ml of 70 hot water to wash, after finishing collecting the mother liquor and this washing water, The filter cake was continuously washed with water until it was neutral and then dried to obtain 24.1 g of a finished product of solvent violet 13 with a main content of 97.83%. Put the last batch of mother liquor + first wash water into a 500ml autoclave at once, 1,4-dihydroxyanthraquinone 14g, 1,4-dihydroxyanthraquinone leuco 4.6g, flake base 2g, and p-toluidine 7.2g. 0.2g of boric acid, 0.05g of phase transfer catalyst. After stirring for 0.5h, the temperature is slowly raised to 115 in a sealed seal. After 3h of heat preservation, the tracking detection starts, and the temperature is stopped at 6h. At this time, the content of 1,4-di-p-tolylaminoanthraquinone is 0.58 %, the main content of the product is 57.2%, and the temperature is reduced to 80°C,Relieve the pressure, filter at this temperature, then add 20ml of 70 hot water to wash, After the mother liquor and the washing water were collected, the filter cake was washed until it was neutral and dried to obtain 24.9 g of the finished product of purple 13 with a main content of 98.44% and a single batch yield of 97.3%. Comparing the product with Solvent Violet 13 standard sample, DE is 0.95, DC is 0.29, DC is 0.88, and intensity is 100.15. After continuous application of this series of batches, the main content is stable at 96.8% to 98.5%, and the total yield is 95.33%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping