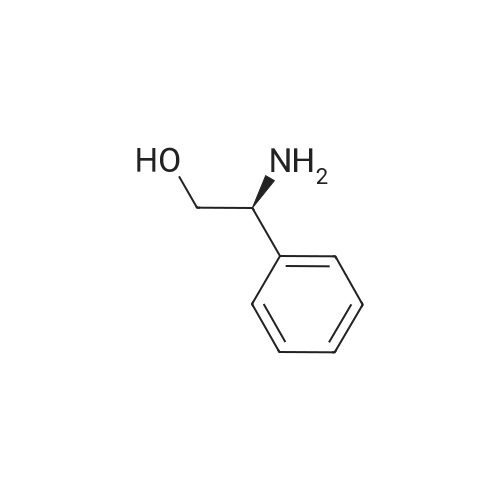
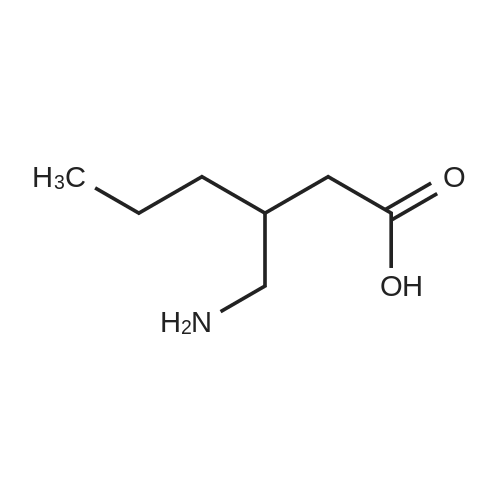
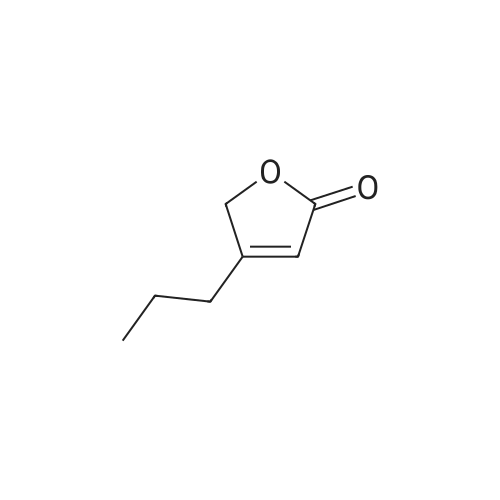
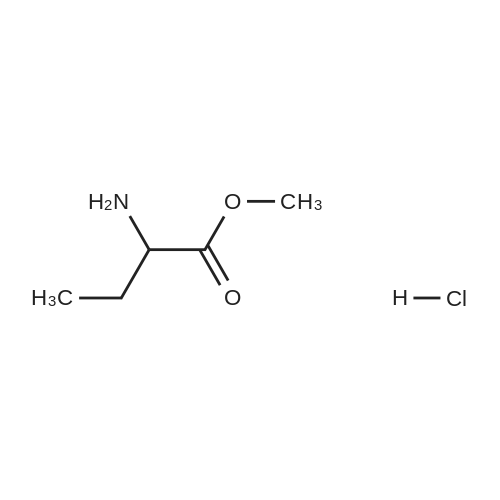
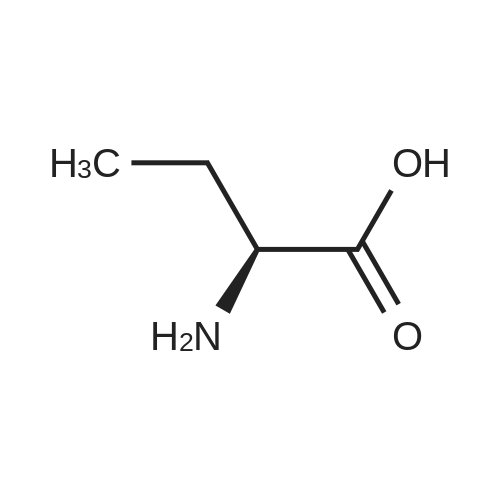
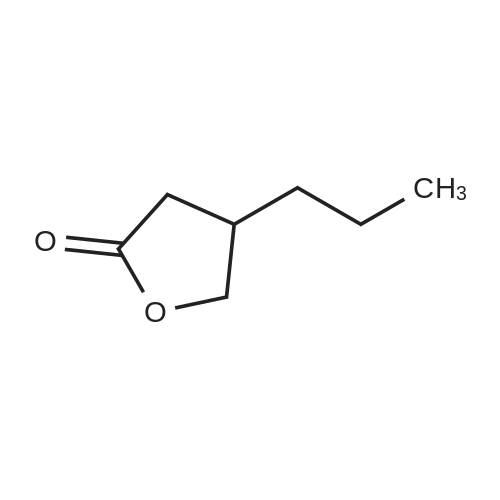
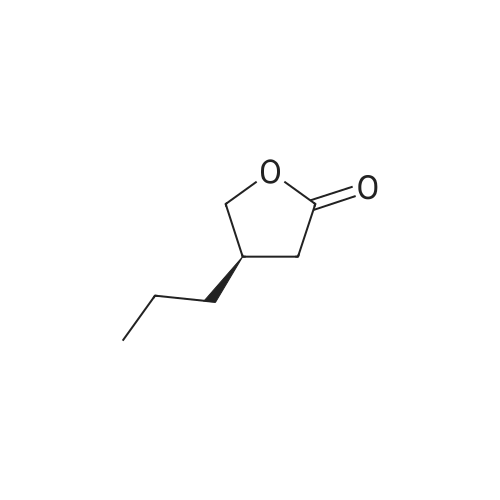
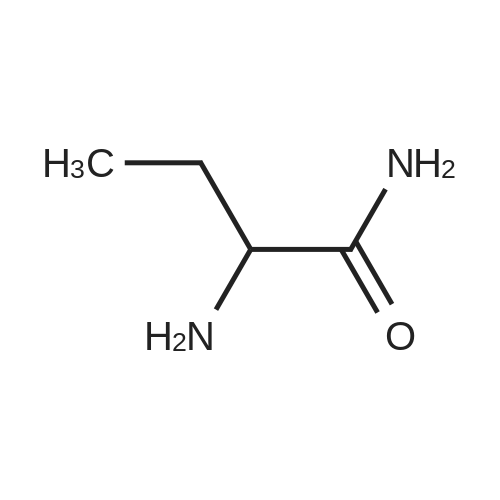
| 81.2% |
|
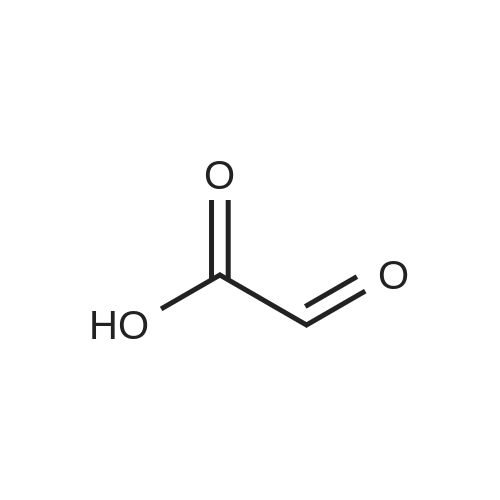
Add 500 ml of four-necked flask with heptane (140 mL) and morpholine (43 mL) for mechanical stirring.50 mL of glyoxylic acid was added at t = 0 C, and reacted at 20 C for 1 h. Add 50 mL of n-pentanal,Then t = 43 C, 20h. After the reaction was over, the reaction was quenched with 65 mL of concentrated hydrochloric acid.Stir at room temperature for 2 h. The mixture was separated, and the aqueous phase was washed with EtOAc (EtOAc).50.6 g of brown oil was obtained.Yield 81.2% |
| 55% |
With piperidine hydrochloride; In 1,4-dioxane; water; for 18h;Inert atmosphere; Reflux; |
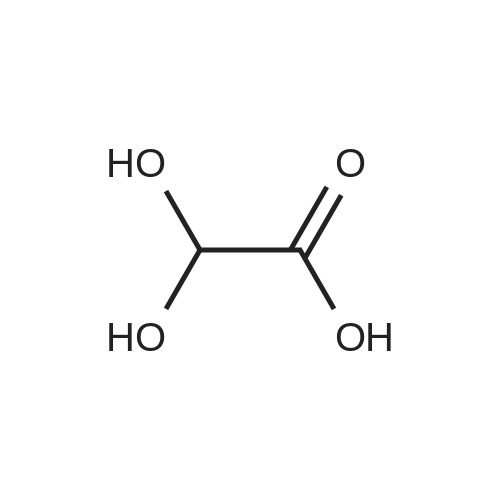
Glyoxilic acid monohydrate (4.55 g, 49.42 mmol) and piperidine hydrochloride (6.30 g, 51.80 mmol) were added to a solution of pentanal (5 mL, 47.02 mmol) in 7:1 dioxane:water (25 mL) under nitrogen. The mixture was heated at reflux for 18 h and, after cooling to room temperature, diluted with 2 M HCl (100 mL) and extracted with diisopropyl ether (3 × 30 mL). The first extract was discharged, while the other two were combined and extracted with 2 M HCl (3 × 30 mL). These three acidic extracts were combined with the reaction mixture, which had been previously diluted with 2 M HCl, and washed with diisopropyl ether, and the whole aqueous layer was extracted with DCM (3 × 80 mL). The DCM extracts were concentrated to give 4a (3.68 g, 55%) as an oil. 1H NMR (300 MHz, CDCl3) delta 6.00 (s, 1H), 5.84 (t, J = 1.8 Hz, 1H), 4.65 (bs, 1H, exchange with D2O), 2.25-2.52 (m, 2H), 1.52-1.77 (m, 2H), 1.00 (t, J = 7.6 Hz, 3H). 13C NMR (75 MHz, CDCl3) delta 172.3, 170.3, 117.2, 99.4, 29.6, 19.9, 13.7. Anal. calcd. for C7H10O3: C, 59.14; H, 7.09. Found: C, 58.98; H, 7.13. |
|
|
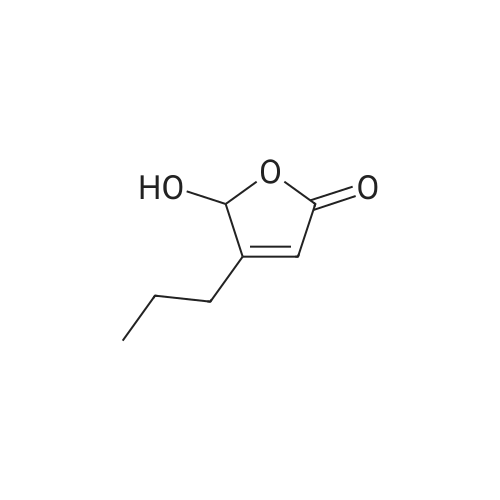
Example 1: Synthesis of 5-hydroxy-4-/i-propyl -5//-furan-2-one [Va] (J. Org. Chem. 1981, 46, 4889-4894); tt-Heptane (394 mL) and morpholine (127.5 mL) are introduced in a reactor while stirring. The mixture is cooled to 0 C and glyoxylic acid (195 g, 150 mL, 50 wt % in water) is added. The mixture is heated to 20 C during 1 hour and then 77-valeraldehyde (148.8 mL) is added. The reaction mixture is heated at 45 C during 20 hours. After cooling down to 20 C, a 37 % aqueous solution of hydrochloric acid (196.9 mL) is slowly added to the mixture, which is then stirred during 2 hours. After removal of the heptane phase, the aqueous phase is washed three times with heptane. Di- w -propyl ether is added to the aqueous phase. The organic phase is removed and the aqueous phase further extracted with di-z'so-propyl ether (2x). The di-wo-propyl ether layers are combined, washed with brine and then dried under reduced pressure. After evaporation of the solvent, 100.0 g of 5-hydroxy-4-«-propyl-5H-furan-2-one are obtained as light brown oil.FTIR (neat): 3367, 1735 cm"1. NMR (CDC13, 200 MHz): delta 0.93-1.00 (t, 3H), 1.56-1.67 (q, 2H), 2.31-2.43 (q, 2H), 5.81 (s, 1H), 6.02 (s, 1 H).MS (EI): C7H,o03: 142.06; [M+H]+: 142.93. |
|
|
Example 1: Synthesis of 5-hydroxy-4-/i-propyl-5H-furan-2-one [XI](Reference: J. Org. Chem 1981, 46, 4889-4894)Heptane (394 mL) and morpholine (127.5 mL) are introduced in a reactor while stirring. The mixture is cooled to 0C and glyoxylic acid (195 g, 150 mL, 50 wt% in water) is added. The mixture is heated to 20C during 1 hour and then n-valeraldehyde (148.8 mL) is added. The reaction mixture is heated at 45C during 20 hours. After cooling down to 20C, a 37% aqueous solution of hydrochloric acid (196.9 mL) is slowly added to the mixture, which is then stirred during 2 hours.After removal of the heptane phase, the aqueous phase is washed three times with heptane. Di-z'so-propyl ether is added to the aqueous phase. The organic phase is removed and the aqueous phase further extracted with di-z'so-propyl ether (2x). The diisopropyl ether layers are combined, washed with brine and then dried under reduced pressure. After evaporation of the solvent, 100.0 g of 5-hydroxy-4-n-propyl-5H-furan-2-one are obtained as light brown oil.FTIR (neat): 3367, 1735 cm"1.1H NMR (CDC13, 200 MHz): delta 0.93-1.00 (t, 3H), 1.56-1.67 (q, 2H), 2.31-2.43 (q, 2H), 5.81 (s, 1H), 6.02 (s, 1H).MS (EI): C7H10O3: 142.06; [M+H]+: 142.93. |
|
|
Example 1 : Synthesis of 5-hydroxy-4-n-propyl-5H-furan-2-one (Reference: J. Org. Chem 1981 , 46, 4889^894)Heptane (394 ml_) and morpholine (127.5 ml_) were added into the reactor while stirring. The mixture was cooled to 0C and glyoxylic acid (195 g, 150 ml_, 50 wt% in water) was added and after complete addition reaction mixture was heated to 20C and stirred for 1 h. To the above reaction mixture n-valeraldehyde (148.8 ml_) was added slowly and after complete addition reaction mixture was heated to 45C and stirred for 20 h. After which reaction mixture was cooled to 20C and 37% aqueous solution of hydrochloric acid (196.9 ml_) was slowly added to the mixture, and further stirred for 2 hours.After removal of the heptane phase, the aqueous phase was washed three times with heptane. Aqueous phase is extracted with di-/so-propyl ether (3x250 ml_). The combined di /so-propyl ether layers was washed with brine and solvent was evaporated under reduced pressure to obtain 100.0 g of 5-hydroxy-4-n-propyl-5/-/-furan-2-one as light brown oil.FTIR (neat): 3367, 1735 cm 1.1H NMR (CDCI3, 200 MHz): delta 0.93-1.00 (t, 3H), 1.56-1.67 (q, 2H), 2.31-2.43 (q, 2H), 5.81 (s, 1 H), 6.02 (s, 1 H).MS (El): C7H10O3: 142.06; [M+H]+: 142.93. |
|
|
Example 1 Synthesis of 5-hydroxy-4-n-propyl-5H-furan-2-one [XI](Reference: J. Org. Chem. 1981, 46, 4889-4894) Heptane (394 mL) and morpholine (127.5 mL) are introduced in a reactor while stirring. The mixture is cooled to 0 C. and glyoxylic acid (195 g, 150 mL, 50 wt % in water) is added. The mixture is heated to 20 C. during 1 hour and then n-valeraldehyde (148.8 mL) is added. The reaction mixture is heated at 45 C. during 20 hours. After cooling down to 20 C., a 37% aqueous solution of hydrochloric acid (196.9 mL) is slowly added to the mixture, which is then stirred during 2 hours. After removal of the heptane phase, the aqueous phase is washed three times with heptane. Di-iso-propyl ether is added to the aqueous phase. The organic phase is removed and the aqueous phase further extracted with di-iso-propyl ether (2*). The diisopropyl ether layers are combined, washed with brine and then dried under reduced pressure. After evaporation of the solvent, 100.0 g of 5-hydroxy-4-n-propyl-5H-furan-2-one are obtained as light brown oil. FTIR (neat): 3367, 1735 cm-1. 1H NMR (CDCl3, 200 MHz): delta 0.93-1.00 (t, 3H), 1.56-1.67 (q, 2H), 2.31-2.43 (q, 2H), 5.81 (s, 1H), 6.02 (s, 1H). MS (EI): C7H10O3: 142.06; [M+H]+: 142.93. |
|
In neat (no solvent); at 90 - 180℃; for 0.0833333h; |
Pure glyoxilic acid monohydrate was continuously introduced at T=90C in a plug flow reactor at a flowrate equal to 0.53m1/mn (density = 1 .4 at T=90C) with a flowrate of pure valeraldehyde equal to 0.71 mI/mn (Density = 0,81 at T=25C) in order to get a ratio glyoxylic acid / valeraldehyde = 1.2. The residence time in the reactor is 5mn atT= 18000. This reaction is done without any solvent and catalyst: it is a neat reaction The volume of reactor, made of Hastelloy C is close to 6.2m1. We obtained a yield of hydroxyfuranone equal to 88% with 5.5% of Form E, either a potential yield close to 93% before the workup. Afterwards, we purified said crude product by classical batch technology to remove water, and by-products by several extractions with n-heptaneand IPAC (Isopropyl Acetate) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping