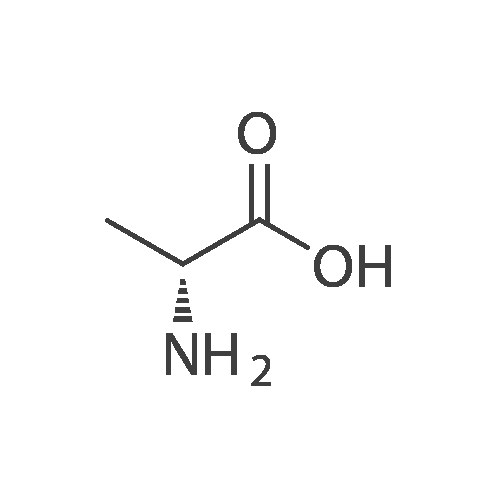
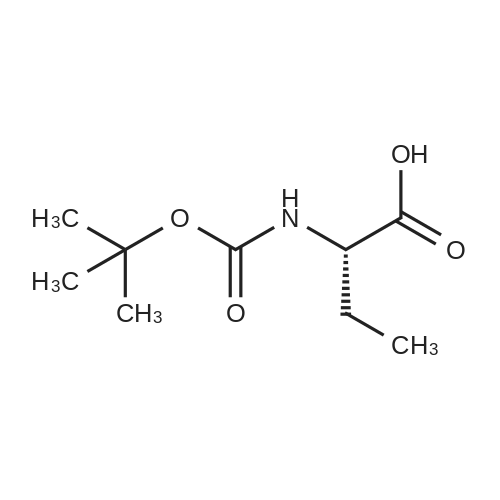
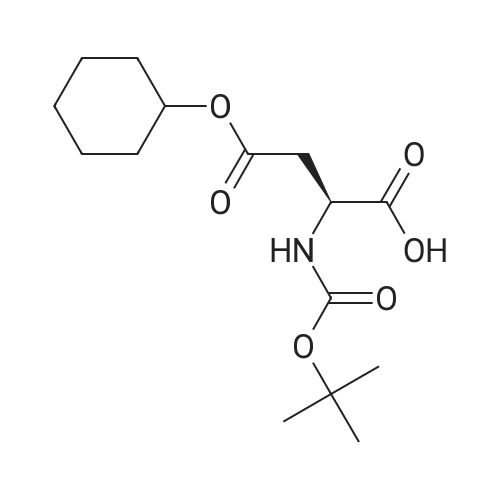
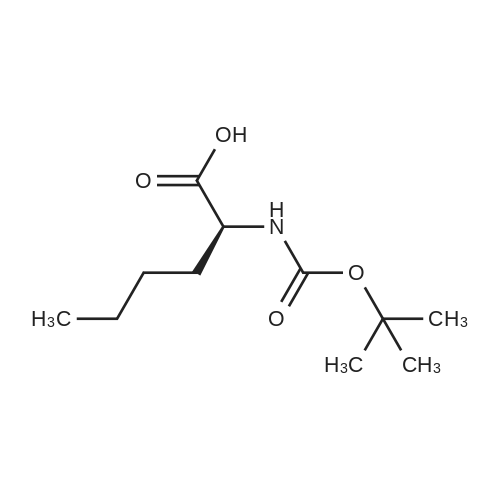
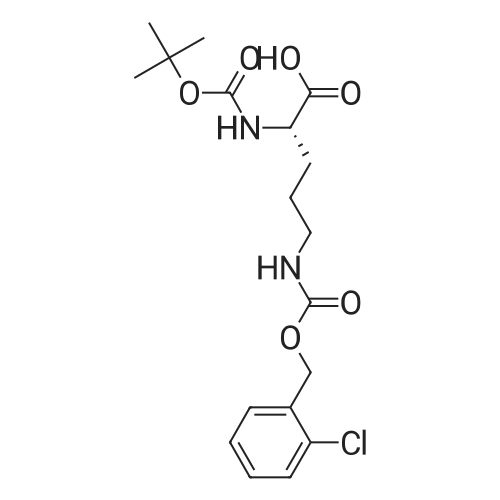
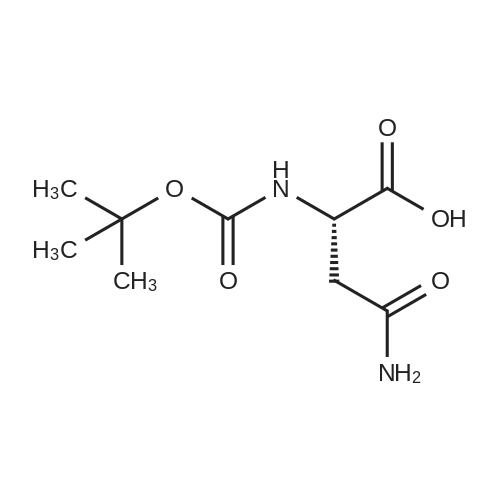
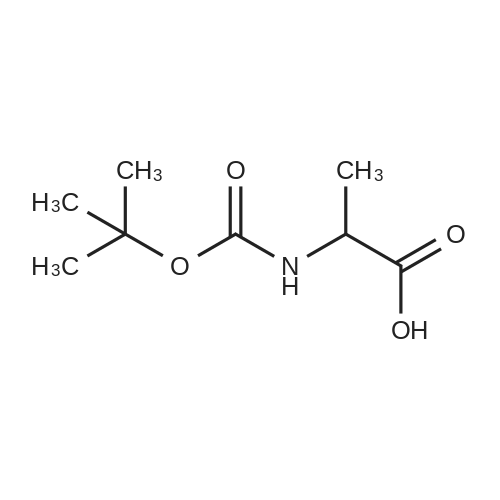
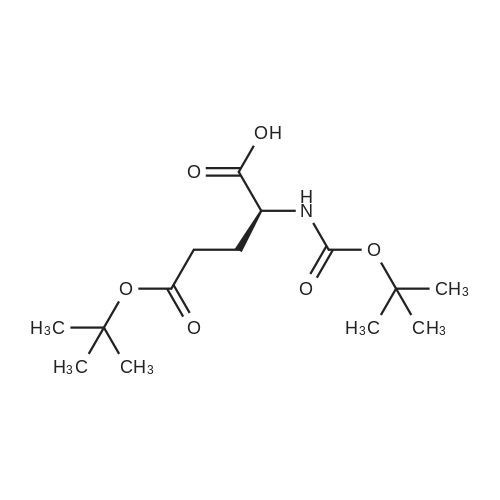
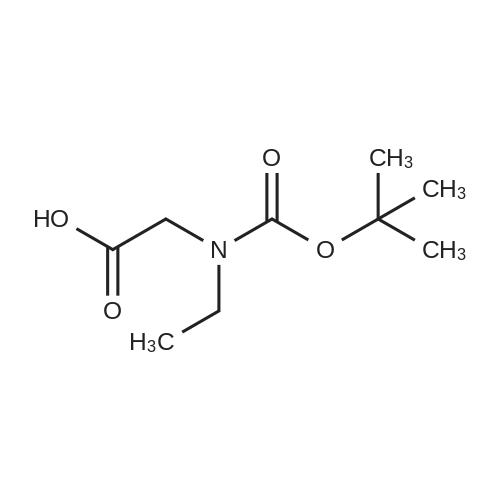
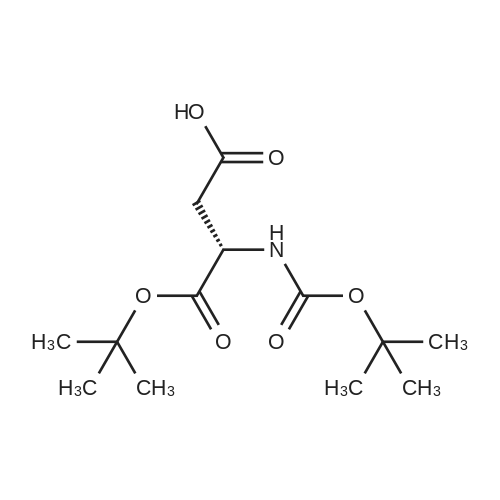
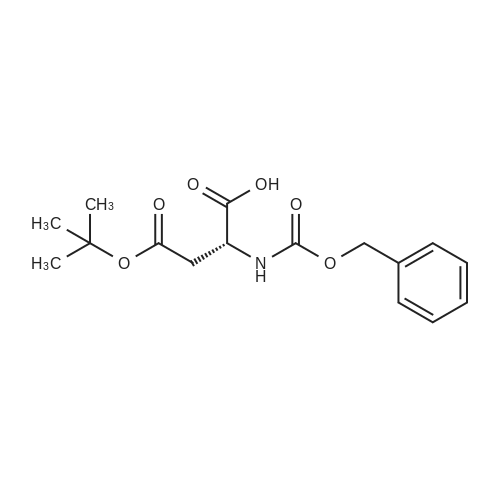
| 90% |
With sodium hydroxide In 1,4-dioxane; water for 20h; Ambient temperature; |
|
| 90.9% |
With triethylamine In tetrahydrofuran; water at 0 - 20℃; |
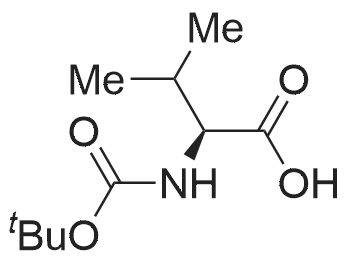
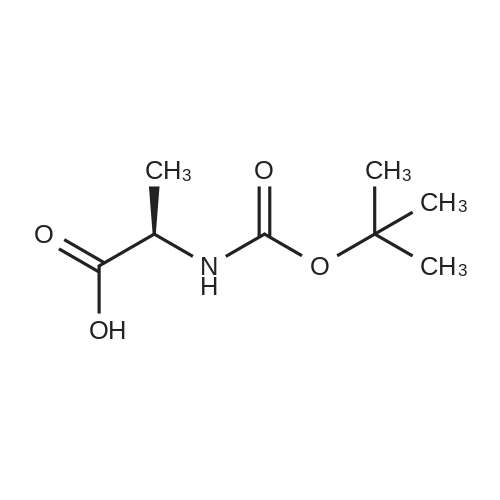
3.1.2. N-tert. Butoxycarbonyl-D-alanine (6a)
To a solution of compound D-alanine (6.23 g, 70 mmol) and triethylamine (11.76 ml, 84mmol) in 40 ml H2O and 20 ml tetrahydrofuran, was added dropwise a solution of 15.28 g(70 mmol) di-tert-butyl dicarbonate in 20 ml tetrahydrofuran at 0 °C. The reaction mixturewas stirred for 0.5 h at 0 °C, then was reacted overnight at room temperature keeping pH 8-9.After cooling to 0 °C the mixture was acidified to pH 2-3 by the dropwise addition of 2 mol/l HCl solution and extracted with ethyl acetate. The organic phase was washedwith saturated brine and dried over anhydrous MgSO4 and concentrated with a rotaryevaporator to get the colorless oil which was stirred in petroleum ether. The white solidwas filtered as the title compound. Yield: 90.9 %; mp: 80.5-81.3 °C. |
| 87% |
With sodium hydroxide In 1,4-dioxane; water at 5 - 20℃; for 72h; |
|
| 82% |
|
|
| 75% |
With triethylamine In 1,4-dioxane; water at 20℃; |
|
| 74% |
With sodium carbonate In tetrahydrofuran; water at 0 - 20℃; for 48h; |
|
| 73% |
With sodium hydroxide In tetrahydrofuran; water at 20℃; for 3h; |
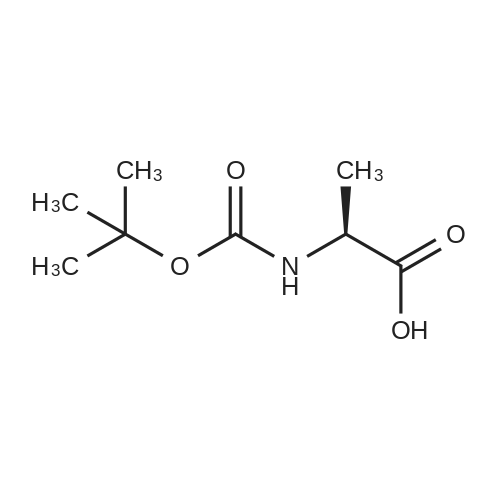
1.1 Step 1 (2R)-2-[(tert-butoxy)carbonyl]amino}propanoic acid (1b)
D-alanine 1a (9.0 g, 0.10 mol) was dissolved in tetrahydrofuran (100 mL)a mixed solution with 10% aqueous sodium hydroxide solution (100 mL),Di-tert-butyl dicarbonate (25 mL, 0.11 mol) was added dropwise.The reaction was carried out for 3 hours at room temperature.Extracted with ethyl acetate (100 mL×2), the aqueous phase was adjusted to pH 2 with 4mol/L hydrochloric acid, and then extracted with dichloromethane/methanol (v/v=10/1, 100mL×2).The combined organic phases were dried over anhydrous sodiumThe title compound 1b was obtained as a white solid (14 g, yield 73%).Can be used directly in the next reaction. |
|
With sodium hydroxide In <i>tert</i>-butyl alcohol for 12h; Ambient temperature; |
|
|
With sodium hydroxide In tetrahydrofuran at 20℃; for 18h; |
|
|
With sodium hydroxide In 1,4-dioxane at 0℃; |
|
|
With sodium hydroxide In water; <i>tert</i>-butyl alcohol at 20℃; |
|
|
Stage #1: D-Alanine; di-<i>tert</i>-butyl dicarbonate With potassium hydroxide In 1,4-dioxane; water at 20℃; for 12h;
Stage #2: With sodium hydrogen sulfate In water; ethyl acetate |
|
|
Stage #1: D-Alanine With sodium hydrogencarbonate In tetrahydrofuran; water for 0.25h;
Stage #2: di-<i>tert</i>-butyl dicarbonate In tetrahydrofuran; water at 20℃; for 4h; |
Intermediate 30To a solution of D-alanine (4.45 g, 50 mmol) in THF (100 mL) and water (50 mL) was added a solution of NaHC03 (4.2 g, 50 mmol) in water (30 mL). After stirring for 15 minutes, a solution ofBoc-anhydride (16.35 g, 75 mmol) in THF (20 mL) was added and the mixture was stirred at room temperature for 4 hours. The solvent was evaporated and 2N HCI was used to adjust the pH=3-4.The mixture was extracted with ethyl actetate (3 times 200 mL) and the combined ethyl acetate layers were washed with brine (50 mL), dried and concentrated. The residue was recrystallized with ethyl acetate/hexane to afford the title compound as a white solid (5 g).HNMR (DMSO-d6): δ ppm 12.38 (1 H, s), 7.1 1 - 7.09 (1 H, d), 3.94 - 3.88 (1 H, m), 1.38 (9H, s), 1.22- 1.21 (3H, d). |
|
With potassium hydroxide In 1,4-dioxane; water at 25℃; for 12h; |
|
| 1.78 g |
Stage #1: D-Alanine; di-<i>tert</i>-butyl dicarbonate With potassium carbonate In methanol; water at 0 - 20℃; for 23.0833h;
Stage #2: With citric acid In methanol; water |
81a Intermediate 81 a: (R)-2-tert-butoxycarbonylamino-propionic acid
To a suspension of D-alanine (1g, 1 1.22 mmol) in water (5.6 mL)/MeOH (2.80 mL) (2:1 ) K2C03 (1.55 g, 1 1.22 mmol) was added at 0-5°C, followed by a solution of Boc20 (2.61 mL, 1 1.22 mmol) in MeOH (2.80 mL) over 5 min. The resulting suspension was stirred for 23 h at rt. The reaction mixture was poured into water and acidified to pH= 3 with aqueous citric acid solution. The white cloudy solution was extracted with DCM (3x). Organic layers were combined, washed with brine, dried over MgS04, filtered and the solvent removed to yield 1.78 g, 9.43 mmol (R)-2- tert-butoxycarbonylamino-propionic acid.1H NMR (400 MHz, DMSO-c 6) δ ppm: 12.14 - 12.63 (m, 1 H), 6.97 - 7.19 (m, 1 H), 3.76 - 4.00 (m, 1 H), 1.38 (s, 9 H), 1.22 (d, J=7.09 Hz, 3 H). |
|
With sodium hydroxide In tetrahydrofuran; water at 20℃; for 0.5h; |
184 (R)-2-(tert-butoxycarbonylamino)propanoic acid (2):
To a stirring solution of (R)-2-aminopropanoic acid SM1 (0.2 g, 2.25 mmol) in THF (10 mL) was added (Boc)20 (0.58 g, 2.7 mmol) and 10% NaOH aq (10 mL), the mixture was stirred at room temperature for 30 mins. The solvent was removed out in vacuo, the residue was diluted with brine and extracted with EtOAc. Combined organic extracts were dried over anhydrous Na2SO4 and concentrated under reduced pressure to obtain cmde product as an off-white solid, which was useddirectly for subsequent step. ‘HNMR (300 MHz, DMSO-d6): 12.44 (s, ‘H), 7.11 (s, 1H), 3.91 (m, 1H), 1.47 (d, J = 3.0 Hz, 3H), 1.37 (s, 9H). |
|
With sodium hydroxide In water at 0 - 20℃; |
1 Example 1 Preparation of compound (VIII)
50 g of D-alanine, 52 g of sodium hydroxide and about 40 mL of water were added to a 1000 mL single-necked flask, and about 150 g of di-tert-butyl dicarbonate was added thereto at 0 ± 10 ° C. After completion of the dropwise addition, the mixture was stirred at room temperature overnight, Methyl-N-methoxyhydrochloride, 130 g of 1-ethyl- (3-dimethylaminopropyl) carbodiimide hydrochloride (EDC.HCl) After the addition, the mixture was stirred overnight at room temperature, and the reaction mixture was extracted with methylene chloride. The dichloromethane was washed once more with water, and the solvent was evaporated to give compound (VIII). |
|
In chloroform |
|
| 9.6 g |
With potassium hydroxide In tetrahydrofuran; water |
2.1 Ν- (tert-butoxycarbonyl) -D-alanine (8a)
(50 mmol) of D-alanine, 3.8 g (55 mmol) of potassium hydroxide and 13.0 g (55 mmol) of di-tert-butyl carbonate were suspended in a mixed solvent of water (200 mL) and THF (20 mL) Followed by stirring to give 9.6 g of N-Boc-D-alanine as a white solid which was used directly in the next esterification reaction without purification. |
| 5.07 g |
Stage #1: D-Alanine With sodium hydroxide In water at 26℃; for 0.166667h;
Stage #2: di-<i>tert</i>-butyl dicarbonate In water at 26℃; for 12h; |
12 Synthesis of N-Boc-D-alanine compound 8, the reaction formula is:
D-alanine (2.5 g, 27.8 mmol) was added to a dry flask.Distilled water (14 mL) and 1 M aqueous sodium hydroxide (139 mL) were added.After stirring at 26 ° C for 10 min, (Boc) 2 O (12.12 g, 55.6 mmol) was added thereto.The reaction was carried out at 26 ° C for 12 hours, and the reaction compound was lowered to 0 ° C.After adding 3M hydrochloric acid to adjust pH 2, the reaction mixture was diluted with 50 mL of ethyl acetate, and then the mixture was extracted with ethyl acetate three times, 70 mL each time, and the organic phase was combined.Dry over anhydrous sodium sulfate. The solvent is distilled off to obtain N-Boc-D-alanine 8,White solid 5.07 g. |
|
Stage #1: D-Alanine With sodium carbonate In 1,4-dioxane; water
Stage #2: di-<i>tert</i>-butyl dicarbonate In 1,4-dioxane; water for 18h; |
General procedure for the preparation of amino acidderivatives
General procedure: The amino acid derivatives were prepared employinga methodology developed by our research group.19Initially, the coupling between the tert-butyloxycarbonyl(Boc) protecting group and amino acids (L-alanine,D,L-alanine, D-alanine, L-valine, L-leucine, L-isoleucineor L-phenylalanine, 1.0 mmol) was done by adding toa solution of 1,4-dioxane/water (40:60 v/v) the aminoacid and 50 mg of Na2CO3. The mixture was stirred untilcomplete dissolution and then Boc2O (1.5 mmol) wasadded. The reaction was kept at magnetic stirring for 18 hand then purified employing literature protocols. For the amide bond formation, the previously preparedBoc amino acids (1.0 mmol) were transferred to a famedriedglassware vial with dichloromethane (5.0 mL) andthen EDC (3-dimethylaminopropyl)-N-ethylcarbodiimide)hydrochloride (1.1 mmol) was added. The reaction stirredat 0 °C for 30 min, and then washed twice with distilledwater. The organic layer was immediately transferred toa vial with the amine nucleophile (2.0 mmol) and the(±)-camphorsulfonic acid organocatalyst (0.1 mmol,10 mol%). After 24 h the dichloromethane was removedand the desired compounds purified by recrystallization,liquid-liquid extraction or column chromatography.Boc deprotection of two of the previously preparedproducts was carried out following literature protocols,21in which the derivatives (1 mmol) and trifluoroacetic acid(22 mmol) were added in dichloromethane (5 mL) and keptunder magnetic stirring for 3 h, affording the salts 33 and34 after solvent removal. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping