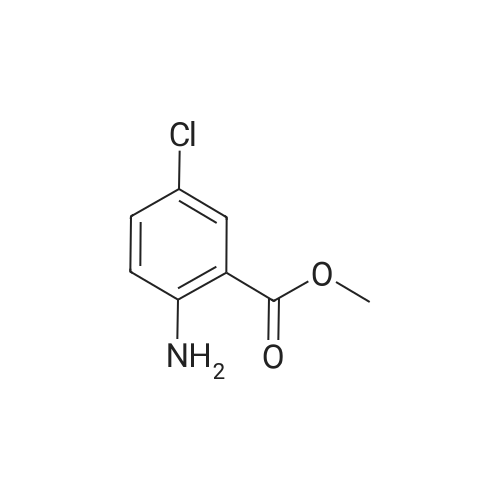
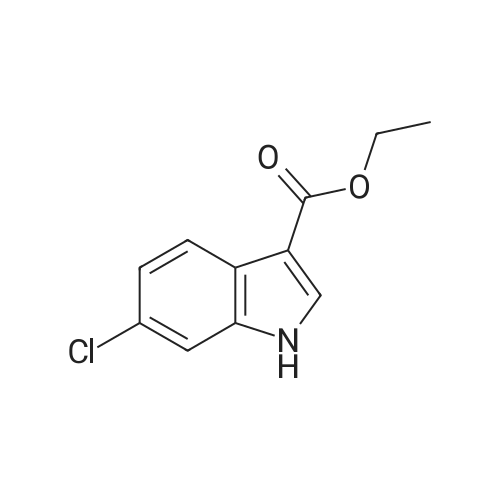
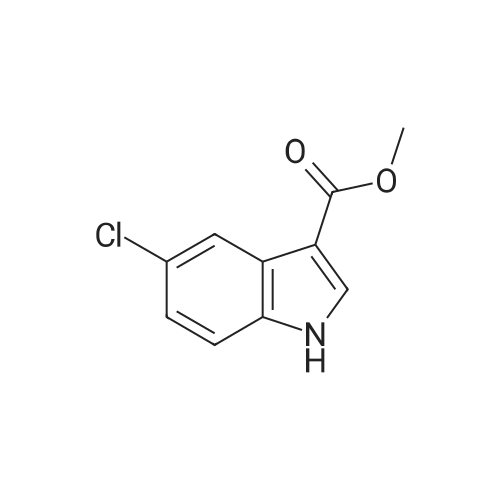
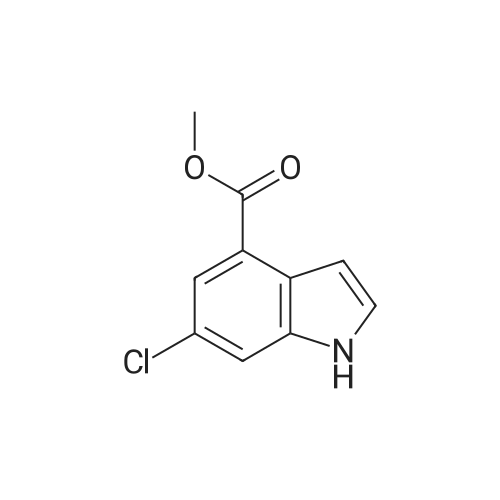
| 75% |
|
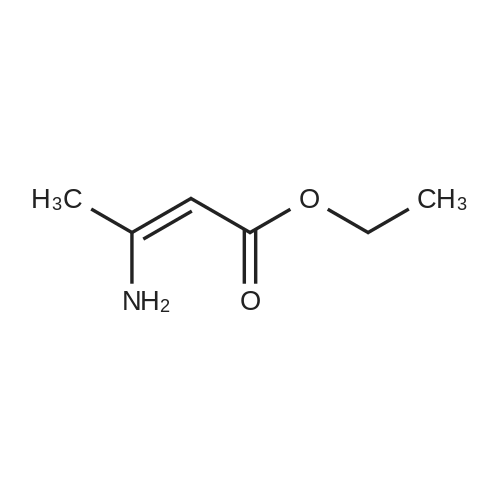
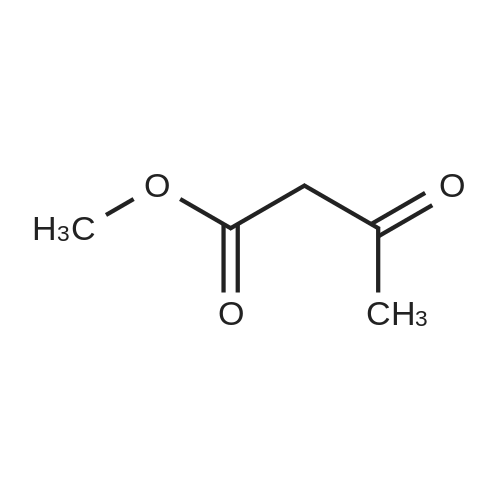
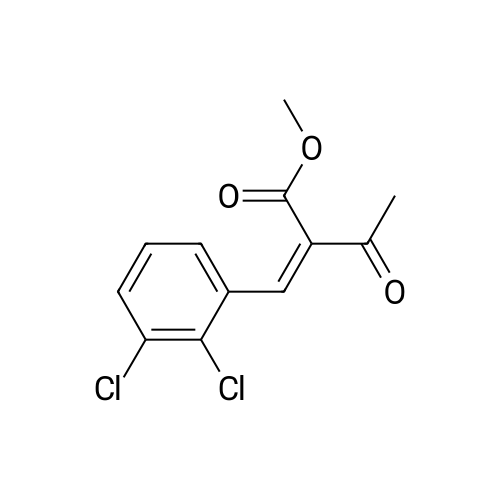
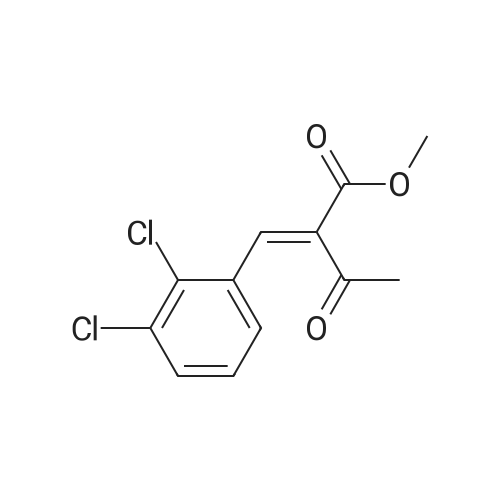
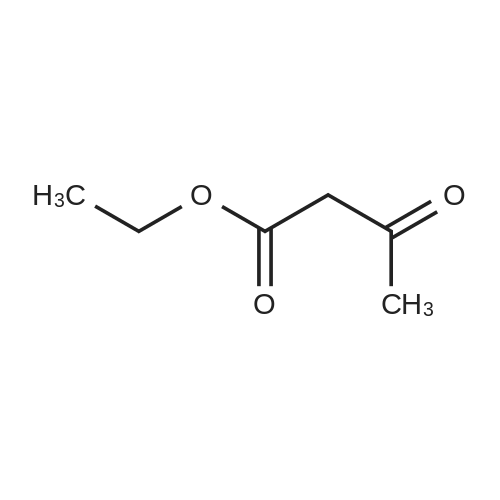
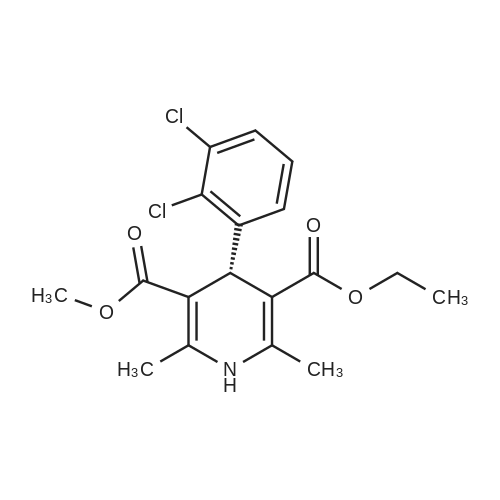
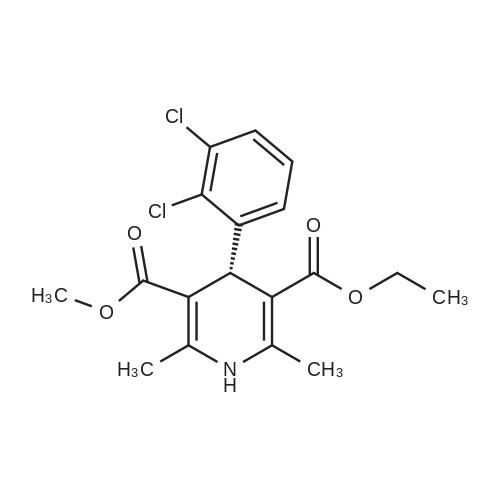
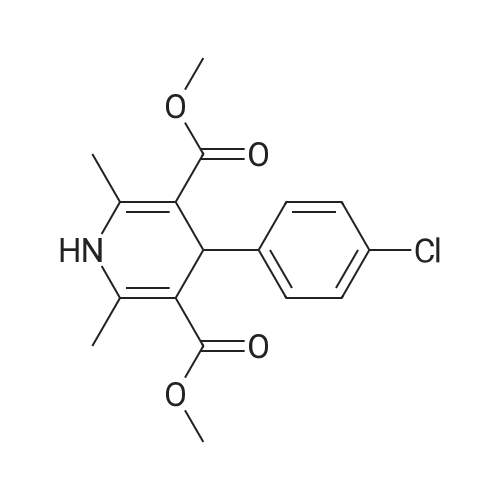
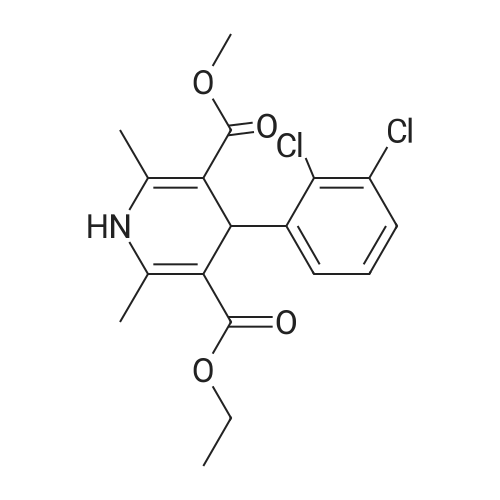
EXAMPLE 1 (method a1, a2) Preparation of 2,6-dimethyl-4-(2,3-dichlorophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid-3-methylester-5-ethylester 2.87 g of 2,3-dichlorobenzylideneacetylacetic acid-methylester and 1.3 g of 3-aminocrotonic acid ethylester were dissolved in 10 mls of t.-butanol. The reaction mixture was allowed to stand at ambient temperature for 4 days, whereupon the t.-butanol was evaporated and the residue was dissolved and was stirred with a small amount of isopropylether, whereby the compound crystallized. After recrystallization from isopropylether pure 2,6-dimethyl-4-(2,3-dichlorophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid-3-methylester-5-ethylester was obtained. M.p. 145 C. Yield 75%. |
| 64.8% |
|
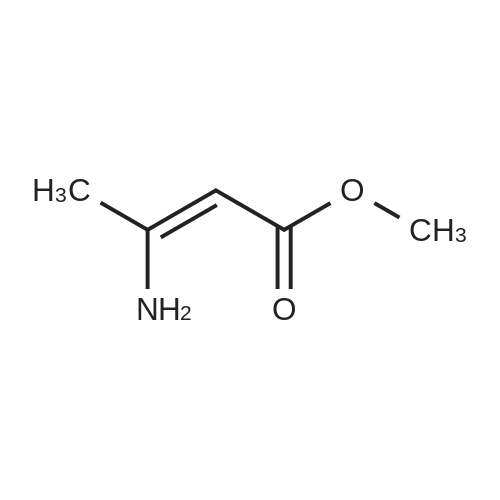
EXAMPLE 2 Preparation of Felodipine Involving Shortened Thermal Period and Subsequent Acid Catalysis with 1N HCl Solution A stirred mixture of 27 g. of dichlorobenzylidine IIb (99.7% pure by HPLC, 98.6 mmol) and 15.7 g. of ethyl 3-aminocrotonate (121.3 mmol) in 25 mL of anhydrous ethanol under an argon atmosphere was rapidly heated to 83 C. (reflux) and the solution maintained at reflux for 1.5 hours. The heating mantle was then removed and the solution temperature was allowed to fall to 75 C. A solution of 15 mL 1N aqueous HCl and 15 mL ethanol was added dropwise over a 5 min. period to the hot solution. At the end of the acid addition the temperature was 48 C. The solution was allowed to continue cooling and crystallization began at 33 C. The reaction mixture was allowed to cool to 25 C., then it was cooled in an ice bath for 45 minutes and then refrigerated over the weekend. The solids were filtered cold and washed with 1:1 v/v ethanol/H2 O (precooled to -20 to -25 C). The pH of the filtrate at the end of the washing was about pH 7. The solid was suction dried under a nitrogen stream then dried under high vacuum at 40 C. overnight to provide 30.6g. of the desired product (HPLC 80.3% pure) 64.8% yield. |
| 59.4% |
|
The solids were dried in vacuo to afford pure Felodipine as a light yellow crystalline solid (56.9 g, 59.4% yield based on ethyl 3-aminocrotonate). |
| 44.5% |
|
EXAMPLE 5 Preparation of Felodipine Involving Shortened Thermal Period Without Subsequent Acid Catalysis A stirred solution of 27 g. of dichlorobenzylidine IIb (99-100% pure, 99 mmol) and 15.9 g (122.7 mmol) of ethyl 3-aminocrotonate in 25 mL of anhydrous ethanol under an argon atmosphere was rapidly heated to 87 C. (reflux) and the solution maintained at reflux for 1.5 hours. The heating mantle was then removed and the solution temperature was allowed to cool to room temperature (30 mins.). Crystallization was observed when the solution temperature reached 60 C. The mixture was then cooled in an ice bath for 30 mins. and then refrigerated over the weekend. The mixture was again cooled in an ice bath and a 1:1 v/v ethanol:water solution (30 mL) was added dropwise. The mixture was then stirred 1 hour in the ice bath, then filtered and the solid washed with 90 mL of ethanol/water (precooled). The solid was suction dried under a nitrogen stream, then dried under high vacuum at 40 C. overnight to provide 27.5 g of the desired product I (HPLC:61.7% pure) 44.5% yield. |
|
|
General Procedure for Preparation Felodipine Compositions |
|
|
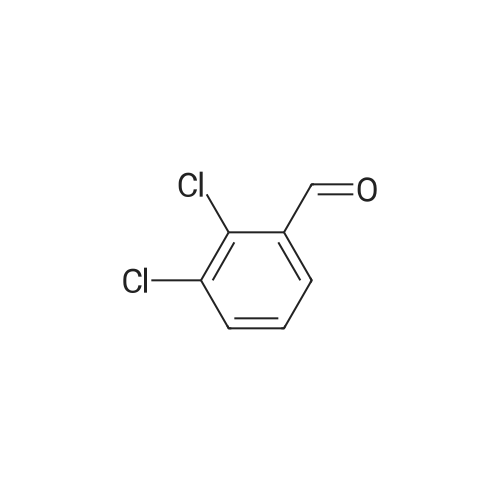
Step B. Synthesis of Felodipine from MBI A 2 L flask was equipped with a mechanical agitator, nitrogen purge, reflux condenser, addition funnel and internal temperature probe and charged with the MBI solids (about 95 g, 0.35 mol, 1.0 equiv) and 266 ml isopropanol and the contents are brought to reflux. A solution of about 0.90 equiv of ethyl 3-aminocrotonate (about 40.4 g EAC for 95 g MBI charge, 0.31 mol, 0.9 equiv) in 96 mL of isopropanol was added to the refluxing solution at such a rate that the internal temperature was maintained at reflux (ca. 83 C. or about 10 min addition time). The resulting mixture was kept at reflux for 60-70 min, then the temperature brought down to below 50 C. by (1) removing heat source, (2) adding 96 niL of isopropanol, and (3) vacuum distillation of isopropanol. The vacuum distillation was performed at below 50 C. to remove approximately 200-300 g of overhead. Cyclohexane (155 g) was added to the residue and the distillation continued at below 50 C. to remove an additional 100-150 g of overhead. Another cyclohexane (155 g) portion was added to the residue and the distillation continued at below 50 C. to remove yet another 100-150 g of overhead. A third cyclohexane (780 g) portion was added and the contents brought to atmospheric reflux (ca. 80-85 C.) for about 6 hrs. The slurry was cooled to about 30-35 C. and the crude felodipine isolated by filtration and washed with 50 g cyclohexane. |
|
|
Step B. Synthesis of Felodipine The resulting MBI was dissolved in isopropanol and brought to reflux. Ethyl 3-aminocrotonate (about 100.9 g, 0.78 mole) in isopropanol was added to the MBI at such a rate to maintain the internal temperature at approximately 83 C. the resulting mixture was refluxed for about 1 hour and the temperature then cooled to about 40-50 C. Excess isopropanol was removed maintaining the internal temperature of the reaction at between about 40 and 45 C. Hexane was then added to the residue, removed, and repeated. The temperature was then brought to room temperature. During this time, the oily residue transformed to granular solids. Hexane was decanted off to yield solid felodipine, along with unreacted MBI, symmetrical dimethyl dihydropyridine along, and dichlorobenzaldehyde. |
|
|
(b) Synthesis of Felodipine from MBI A 3-L flask was equipped with a mechanical agitator, nitrogen purge, reflux condenser, addition funnel, and internal temperature probe was charged with the MBI solids (81.7 g, 0.30 mol), isopropanol (217 mL) and the contents are brought to reflux. A solution of ethyl 3-aminocrotonate (31.8 g, 0.25 mol, 0.83 equiv) in isopropanol (102 mL) was added to the refluxing solution at such a rate that the internal temperature was maintained at reflux (ca. 83-85 C. during 10 minute addition). The resulting mixture was kept at reflux for 70 minutes, then the temperature was brought down to below 50 C. by: (1) removing heat source, (2) adding isopropanol (102 mL), and (3) vacuum distillation of isopropanol. The vacuum distillation was performed at below 50 C. to remove approximately 200-300 grams of distillate. Cyclohexane (170 g) was added to the residue and the distillation continued at below 50 C. to remove and additional 100-200 g of distillate. Another cyclohexane (170 g) portion was added to the residue and the distillation continued at below 50 C. to remove yet another 100-200 g of distillate. A third cyclohexane (800 g) was added and the contents brought to atmospheric reflux (83-85 C.) for about 16 hours. The slurry was cooled to about 30-35 C. and the crude felodipine isolated by filtration and washed with cyclohexane (76 mL). |
|
|
(c) Purification The moist crude felodipine solids were dissolved in methyl t-butylether (MTBE) (810 mL) and hot filtered to clarify the solution. The dissolving flask and filter were rinsed with an additional MTBE (40 mL), which was added to the original hot filtrate. The MTBE solution was placed in a flask equipped with an agitator, nitrogen purge, heat source, temperature probe, distillation head, and receiver. The mixture was concentrated by atmospheric distillation to remove MTBE (internal temperature about 57 C.). Once 200-300 g of distillate was collected, cyclohexane was added in an amount to prepare approximately an 80:20 (w/w) MTBE:cyclohexane solvent system. The resultant solution was cooled and the recrystallized. Felodipine was isolated at about 30-35 C. The recrystallized felodipine solids were further washed with a MTBE/cyclohexane mixture (10 g MTBE+40 g cyclohexane) and cyclohexane (90 mL). |
|
|
Step C. Purification The moist crude felodipine solids were dissolved in about 550 g of methyl tert-butyl ether (MTBE) and hot filtered to clarify the solution. The dissolving flask and filter were rinsed with an additional 30 g of MTBE, which was added to the original hot filtrate. The MTBE solution was placed in a flask equipped with agitator, nitrogen purge, heat source, temperature probe, distillation head and receiver. The mixture was concentrated by atmospheric distillation to remove MTBE (ca. 550 C. internal temperature). Once 100-250 g of distillate have been collected, cyclohexane was added in an amount to prepare approximately an 80:20 (w/w) MTBE:cyclohexane solvent system. The resultant solution was cooled and the recrystallized Felodipine was isolated at about 30-35 C. The recrystallized felodipine solids were further washed with 1*70 g of 20:80 (w/w) MTBE:cyclohexane and 1*70 g of cyclohexane. The solids were dried in vacuo to afford 45-70 g of pure felodipine solids. Theoretical Yield: 172.4 g (MW 383) based on ethyl 3-aminocrotonate |
|
|
EXAMPLE 21 Desulfuration, with Ni-Raney (g 10) suspended in DMF (ml 10), of a solution of KHCO3 (g 0.67) and of 3 g of (-)-S-[6-methyl-3-carboethoxy-5-carbomethoxy-4-(2,3-dichlorophenyl)-1,4-dihydropyridin-2-yl)methyl]-isothiouronium L-0,0'-dibenzoyltartrate in DMF (ml 10) at room temperature for an hour produces g 1.09 of (-)-2,6-dimethyl-3-carboethoxy-5-carbomethoxy-4-(2,3-dichlorophenyl)-1,4-dihydropyridine (m.p. 140-141 C. [alpha]D =-4.4, c=4.1, CH2 Cl2). |
|
|
EXAMPLE 30 By desulphuration of an enantiomerically pure isothiourea and/or enantiomerically pure isothiouronium salt prepared in accordance with one of procedures of examples 28 and 19, and/or by desulphuration of an enantiomerically pure 2-thioalkyl-1,4-dihydropyridine prepared in accordance with one of the procedures of examples 919, starting from one of the isothioureae and/or isothiouronium salts prepared in accordance with procedures of example 19-29, the following enantiomerically pure 1,4-dihydropyridines are prepared: (+)-2,6-dimethyl-3-carboethoxy-5-carbomethoxy-4-(2,3-dichlorophenyl)-1,4-dihydropyridine, m.p. 139-141 C.; [alpha]D =+3.9, [alpha]546 =-4.9, c=4 CH2 Cl2; 2-methyl-3-isopropoxycarbonyl-5-carbomethoxy-4-(2,3-dichlorophenyl)-6-fluoromethyl-1,4-dihydropyridine, enantiomer (+): [alpha]D =+43; enantiomer (-): [alpha]D =-42.8, c=5 DMF; |
|
|
A suitable calcium channel blocker can illustratively be selected from the following list: Arylalkylamines ... benidipine cilnidipine efonidipine elgodipine felodipine isradipine lacidipine lercanidipine ... |
|
|
A suitable calcium channel blocker can illustratively be selected from the following list: Aryklalkylamines ... benidipine cilnidipine efonidipine elgodipine felodipine isradipine lacidipine lercanidipine ... |
|
|
Example 11 600 g of polymer 1, 1000 g of ethylcellulose and 400 g of felodipine (melting point 145 C.) were weighed into a Turbula mixing container and mixed for 10 minutes in the Turbula mixer T10B. Eudragit NE 40D was fed into the extruder via a reciprocating piston pump. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping