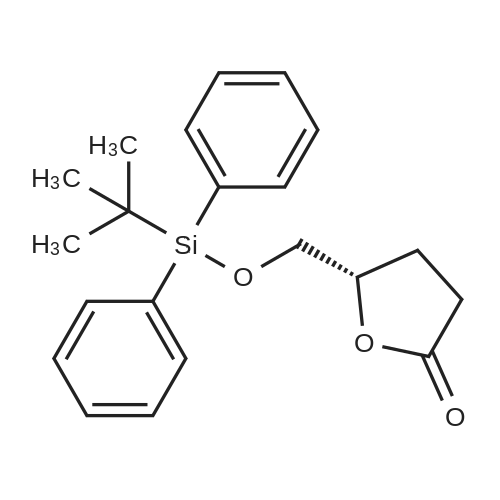
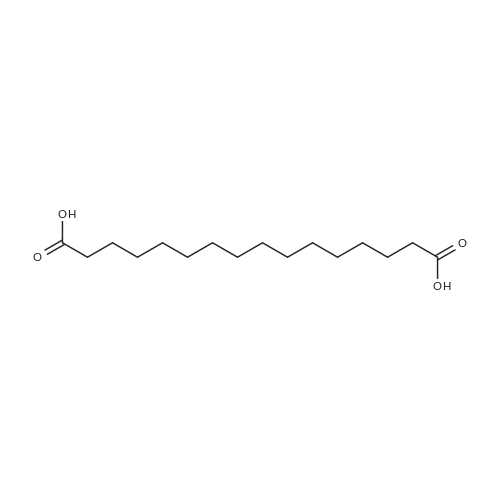
| 58% |
With benzotriazol-1-ol; O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃; for 84h;Product distribution / selectivity; |
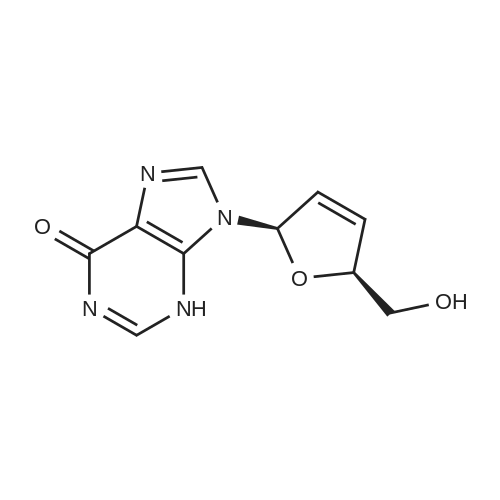
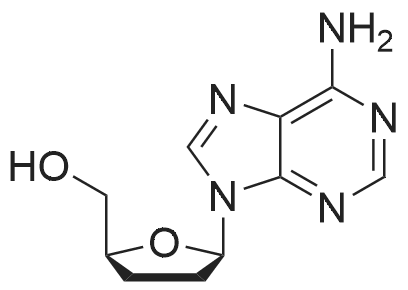
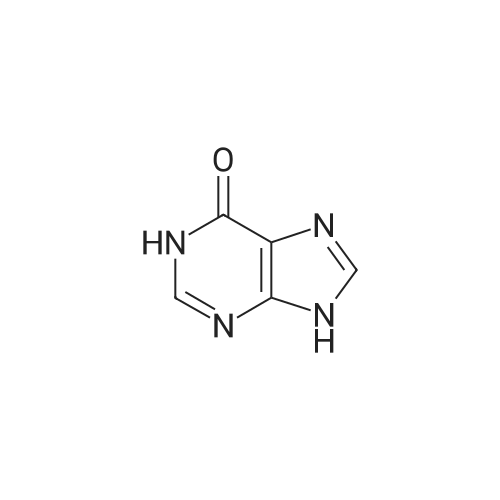
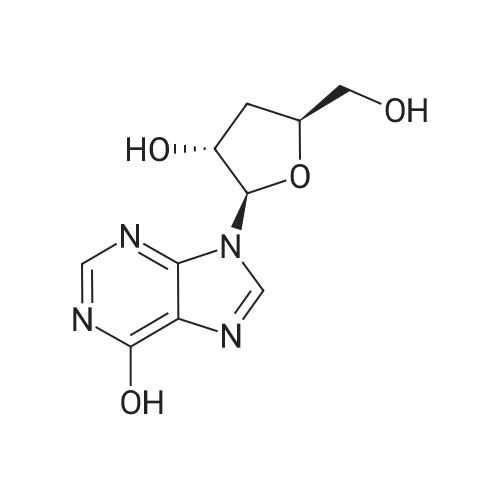
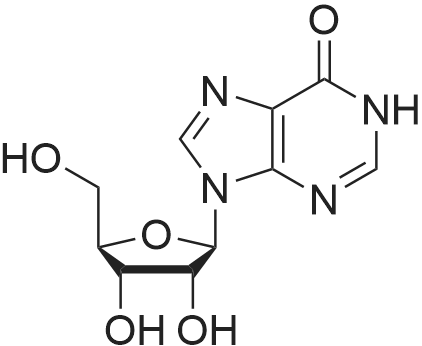
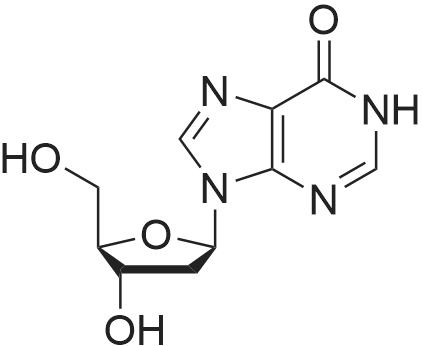
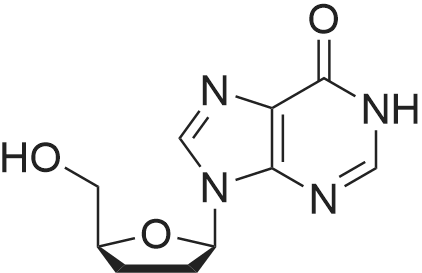
28 mg of N-hydroxybenzotriazole (0.18 mmol), 36 mg of didanosine (ddI, 0.15 mmol), 70 mg of O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluoroborate (0.18 mmol) and finally 62 mg of diisopropylethylamine (0.5 mmol) were added to a solution of 31 mg of (4,8,13,17,21-pentamethyl-docosa-4,8,12,16,20-pentaenoic acid (SqCOOH, 0.15 mmol) in anhydrous dimethylformamide (1.2 ml). The mixture was stirred for 84 hours at 20 C. in a nitrogen atmosphere, then concentrated under reduced pressure (0.05 Torr). The residue was taken up in 5 ml of a saturated aqueous solution of sodium bicarbonate, and extracted with ethyl acetate (3×10 ml). The organic phase was washed with an aqueous NaCl solution, dried over MgSO4 and concentrated under vacuum. The residue was chromatographed over silica gel (CH2Cl2/MeOH: 92/8) to produce 37 mg of 5'-squalenoyldidanosine (Yield 58%) in the form of a colorless amorphous solid.IR (cm-1) 3550-2700, 2921, 2856, 1734, 1691, 1590, 1548, 1449, 1380, 1261.1H NMR (200 MHz, CDCl3) delta: 13.0 (s broad, 1H), 8.18 (s, 1H), 8.08 (s, 1H), 6.38 (t, J=4.2 Hz, IH), 5.17-5.00 (m, 5H), 4.40-4.20 (m, 3H), 2.60-1.90 (m, 24H), 1.67 (s, 3H), 1.60 (s broad, 15H).13C NMR (50 MHz, CDCl3) delta: 173.27 (CO2), 159.20 (CO), 148.34 (C), 144.3 (CH) 138.60 (CH), 135.23 (C), 135.03 (C), 135.00 (C), 133.09 (C), 131.31 (C), 125.56 (CH), 125.38 (C), 125.54 (CH), 124.53 (CH), 124.40 (2 CH), 85.94 (CH), 79.60 (CH), 65.07 (CH2), 39.86 (CH2), 39.83 (CH2), 39.68 (CH2), 34.67 (CH2), 33.12 (CH2), 33.01 (CH2), 28.39 (CH2), 28.38 (CH2), 29.9 (CH2), 26.83 (CH2), 26.79 (CH2), 26.28 (CH2), 25.77 (CH3), 17.77 (CH3), 16.51 (2 CH3), 16.10 (CH3), 16.00 (CH3).The same compound could be obtained in a yield of 10% using EDCI as the coupling agent, while condensation between the squaloyl acid chloride and dI produced it in a yield of 15%.The particle size was determined using the protocol described in example 2. The hydrodynamic diameter mean was 152 nm, measured with a standard deviation of 34.4 nm and a polydispersity index of 0.1. |
| 10% |
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;Product distribution / selectivity; |
28 mg of N-hydroxybenzotriazole (0.18 mmol), 36 mg of didanosine (ddI, 0.15 mmol), 70 mg of O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluoroborate (0.18 mmol) and finally 62 mg of diisopropylethylamine (0.5 mmol) were added to a solution of 31 mg of (4,8,13,17,21-pentamethyl-docosa-4,8,12,16,20-pentaenoic acid (SqCOOH, 0.15 mmol) in anhydrous dimethylformamide (1.2 ml). The mixture was stirred for 84 hours at 20 C. in a nitrogen atmosphere, then concentrated under reduced pressure (0.05 Torr). The residue was taken up in 5 ml of a saturated aqueous solution of sodium bicarbonate, and extracted with ethyl acetate (3×10 ml). The organic phase was washed with an aqueous NaCl solution, dried over MgSO4 and concentrated under vacuum. The residue was chromatographed over silica gel (CH2Cl2/MeOH: 92/8) to produce 37 mg of 5'-squalenoyldidanosine (Yield 58%) in the form of a colorless amorphous solid.IR (cm-1) 3550-2700, 2921, 2856, 1734, 1691, 1590, 1548, 1449, 1380, 1261.1H NMR (200 MHz, CDCl3) delta: 13.0 (s broad, 1H), 8.18 (s, 1H), 8.08 (s, 1H), 6.38 (t, J=4.2 Hz, IH), 5.17-5.00 (m, 5H), 4.40-4.20 (m, 3H), 2.60-1.90 (m, 24H), 1.67 (s, 3H), 1.60 (s broad, 15H).13C NMR (50 MHz, CDCl3) delta: 173.27 (CO2), 159.20 (CO), 148.34 (C), 144.3 (CH) 138.60 (CH), 135.23 (C), 135.03 (C), 135.00 (C), 133.09 (C), 131.31 (C), 125.56 (CH), 125.38 (C), 125.54 (CH), 124.53 (CH), 124.40 (2 CH), 85.94 (CH), 79.60 (CH), 65.07 (CH2), 39.86 (CH2), 39.83 (CH2), 39.68 (CH2), 34.67 (CH2), 33.12 (CH2), 33.01 (CH2), 28.39 (CH2), 28.38 (CH2), 29.9 (CH2), 26.83 (CH2), 26.79 (CH2), 26.28 (CH2), 25.77 (CH3), 17.77 (CH3), 16.51 (2 CH3), 16.10 (CH3), 16.00 (CH3).The same compound could be obtained in a yield of 10% using EDCI as the coupling agent, while condensation between the squaloyl acid chloride and dI produced it in a yield of 15%.The particle size was determined using the protocol described in example 2. The hydrodynamic diameter mean was 152 nm, measured with a standard deviation of 34.4 nm and a polydispersity index of 0.1. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping