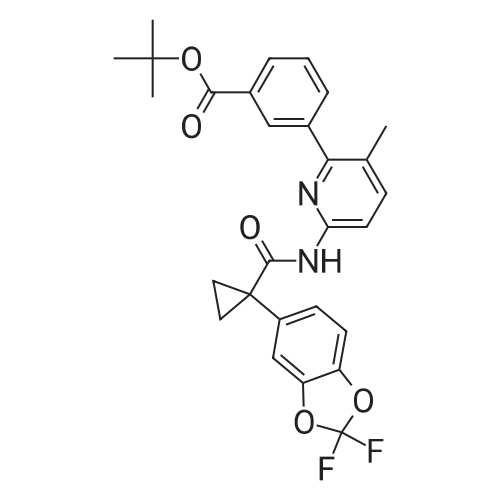
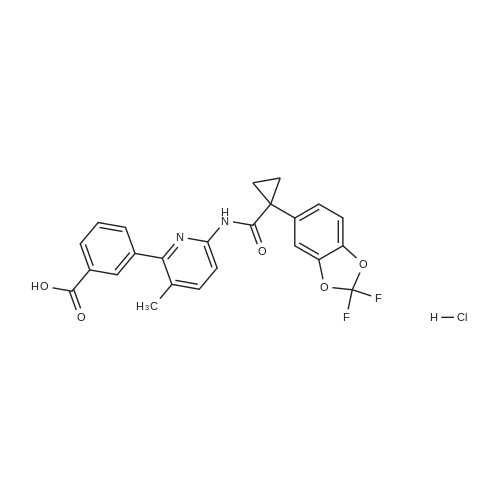
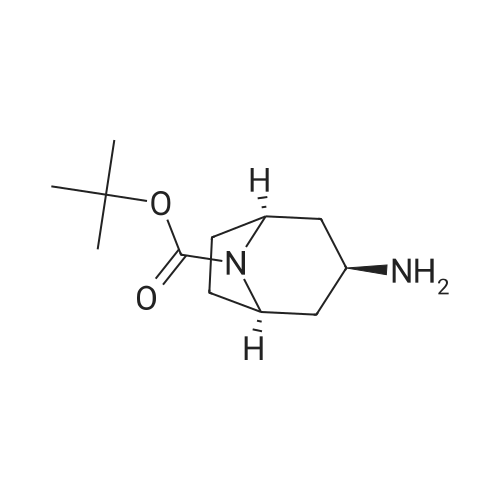
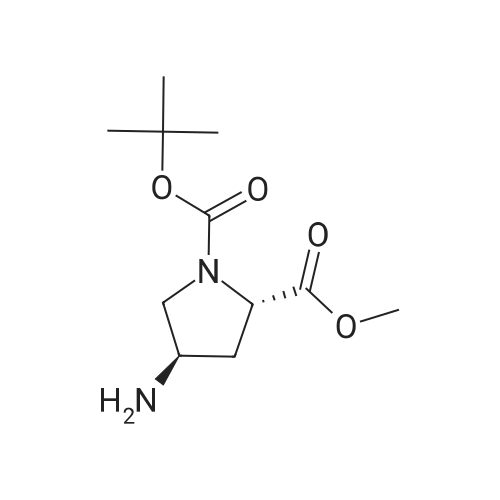
| 84% |
|
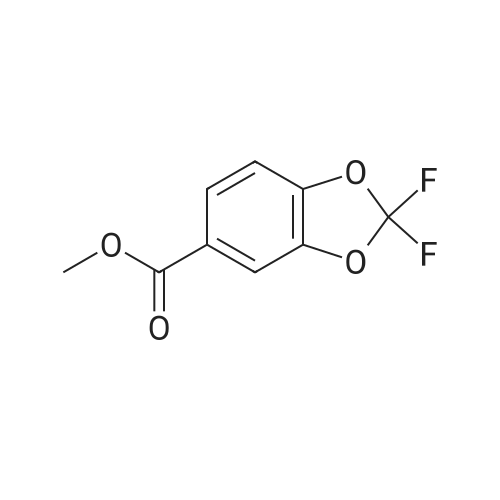
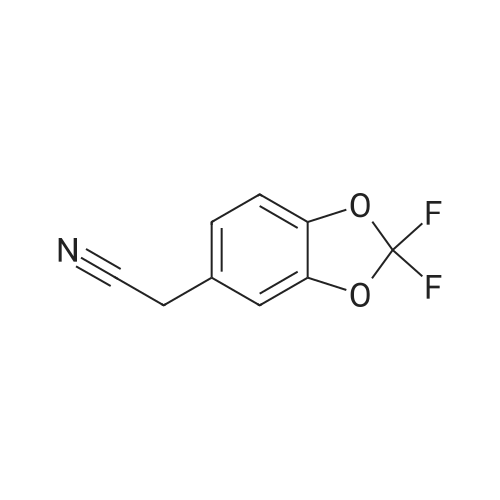
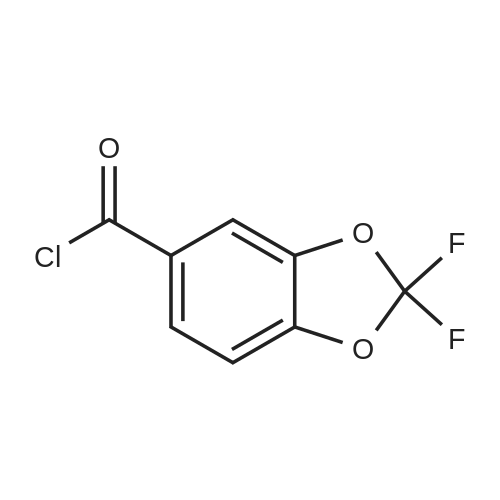
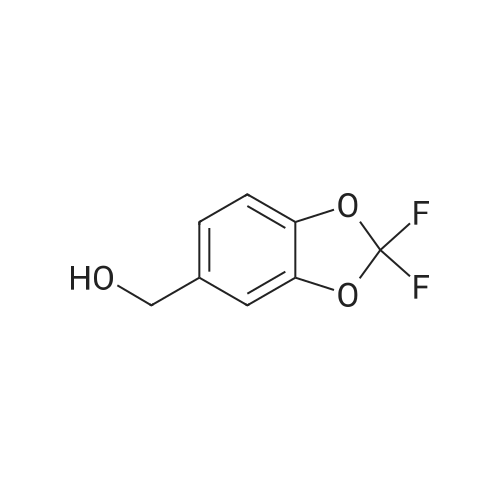
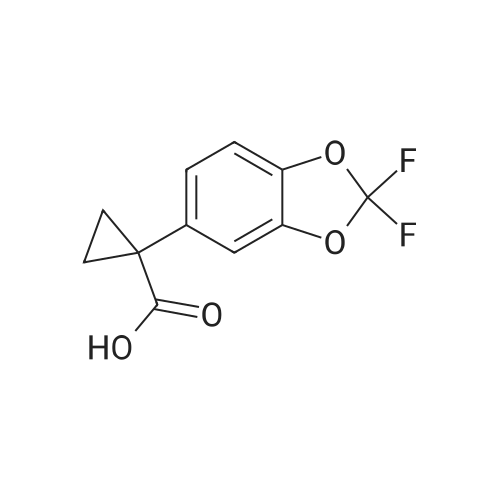
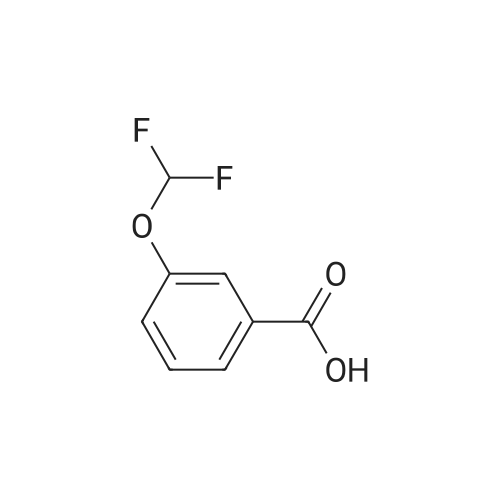
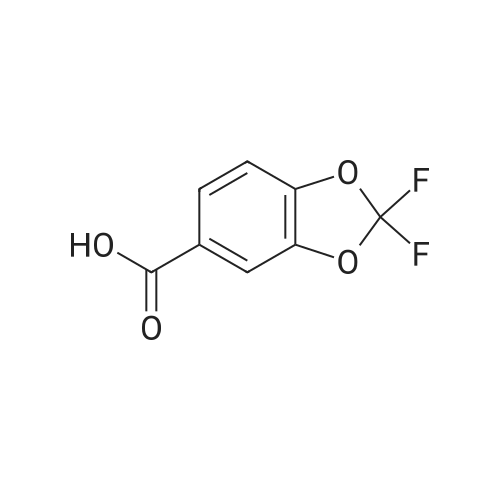
Under nitrogen atmosphere, at 0 C, to a stirred suspension of LiAlH4 (2.0 M THF solution, 6.94 mL,13.88 mmol) in dry THF (29 mL), <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (0.70 g, 3.46mmol) in dry THF (8.0 mL) was added dropwise. The resulting reaction mixture was stirred at roomtemperature for 4 h, and then cooled to 0 C. H2O (1.5 mL) was slowly and cautiously added, followedby 3.0 M KOH solution (1.0 mL) and additional H2O (1.0 mL). The mixture was stirred at 0 C for 30min and then filtered off. The organic layer was dried over Na2SO4, filtered and concentrated todryness, affording the title compound (0.57 g, 84%), which was used in the next step without anyfurther purification. Rt = 1.99 min. 1H NMR (DMSO-d6): delta 7.35-7.29 (m, 2H), 7.14 (d, 1H, J = 8.2Hz), 5.33 (t, 1H, J = 5.7 Hz), 4.49 (d, 2H, J = 5.7 Hz). |
|
|
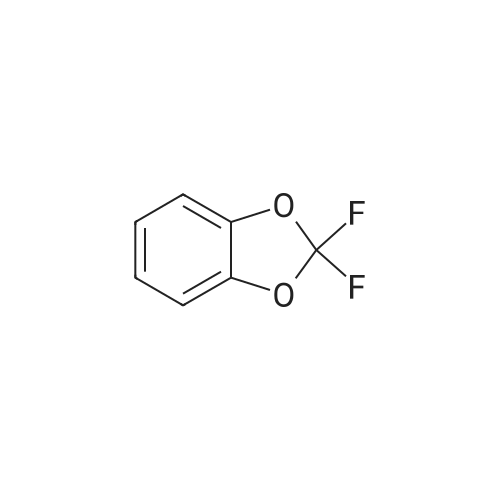
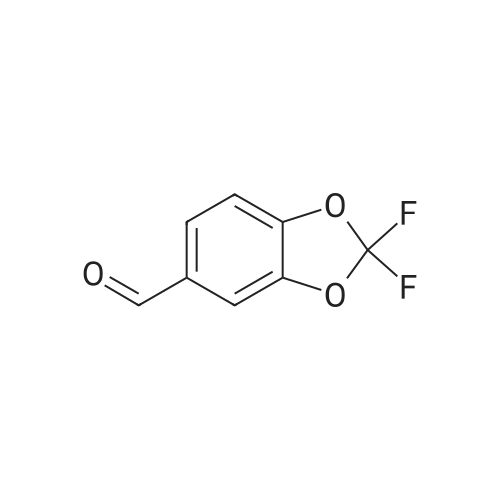
Commercially available 2,2-difluoro-l,3-benzodioxole-5-carboxylic acid (1.0 eq) is slurried in toluene (10 vol). Vitride (2 eq) is added via addition funnel at a rate to maintain the temperature at 15-25 0C. At the end of addition the temperature is increased to 40 0C for 2 h then 10% (w/w) aq. NaOH (4.0 eq) is carefully added via addition funnel maintaining the temperature at 40-50 0C. After stirring for an additional 30 minutes, the layers are allowed to separate at 40 0C. The organic phase is cooled to 20 0C then washed with water (2 x 1.5 vol), dried (Na2SO4), filtered, and concentrated to afford crude (2,2- difluoro-l,3-benzodioxol-5-yl)-methanol that is used directly in the next step. |
|
|
[00193] Synthesis of (2,2-difluoro-l,3-benzodioxol-5-yl)-methanol.1. Vitride (2 equiv)PhCH3 (10 vol)2. 10% aq (w/w) NaOH (4 equiv) 86-92% yield[00194] Commercially available 2,2-difluoro-l,3-benzodioxole-5-carboxylic acid (1.0 eq) is slurried in toluene (10 vol). Vitride (2 eq) is added via addition funnel at a rate to maintain the temperature at 15-25 C. At the end of addition the temperature is increased to 40 C for 2 h then 10% (w/w) aq. NaOH (4.0 eq) is carefully added via addition funnel maintaining the temperature at 40-50 C. After stirring for an additional 30 minutes, the layers are allowed to separate at 40 C. The organic phase is cooled to 20 C then washed with water (2 x 1.5 vol), dried (Na2S04), filtered, and concentrated to afford crude (2,2-difluoro-l,3- benzodioxol-5-yl)-methanol that is used directly in the next step. |
|
With aq. NaOH; In toluene; |
Synthesis of (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq) is slurried in toluene (10 vol). Vitride (2 eq) is added via addition funnel at a rate to maintain the temperature at 15-25 C. At the end of addition the temperature is increased to 40 C. for 2 h then 10% (w/w) aq. NaOH (4.0 eq) is carefully added via addition funnel maintaining the temperature at 40-50 C. After stirring for an additional 30 minutes, the layers are allowed to separate at 40 C. The organic phase is cooled to 20 C. then washed with water (2*1.5 vol), dried (Na2SO4), filtered, and concentrated to afford crude (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol that is used directly in the next step. |
|
In toluene; |
(2,2-Difluoro-1,3-benzodioxol-5-yl)-methanol Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq) was slurried in toluene (10 vol). Vitride (2 eq) was added via addition funnel at a rate to maintain the temperature at 15-25 C. At the end of addition the temperature was increased to 40 C. for 2 hours (h) then 10% (w/w) aq. NaOH (4.0 eq) was carefully added via addition funnel maintaining the temperature at 40-50 C. After stirring for an additional 30 minutes (min), the layers were allowed to separate at 40 C. The organic phase was cooled to 20 C. then washed with water (2*1.5 vol), dried (Na2SO4), filtered, and concentrated to afford crude (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol that was used directly in the next step. |
|
|
Synthesis of (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq) is slurried in toluene (10 vol). Vitride (2 eq) is added via addition funnel at a rate to maintain the temperature at 15-25 C. At the end of addition the temperature is increased to 40 C. for 2 h then 10% (w/w) aq. NaOH (4.0 eq) is carefully via addition funnel maintaining the temperature at 40-50 C. After stirring for an additional 30 minutes, the layers are allowed to separate at 40 C. The organic phase is cooled to 20 C. then washed with water (2*1.5 vol), dried (Na2SO4), filtered, and concentrated to afford crude (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol that is used directly in the next step. |
|
|
Synthesis of (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq) is slurried in toluene (10 vol). Vitride (2 eq) is added via addition funnel at a rate to maintain the temperature at 15-25 C. At the end of addition the temperature is increased to 40 C. for 2 h then 10% (w/w) aq. NaOH (4.0 eq) is carefully added via addition funnel maintaining the temperature at 40-50 C. After stirring for an additional 30 minutes, the layers are allowed to separate at 40 C. The organic phase is cooled to 20 C. then washed with water (2*1.5 vol), dried (Na2SO4), filtered, and concentrated to afford crude (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol that is used directly in the next step. |
|
With sodium bis(2-methoxyethoxy)aluminium dihydride; In toluene; at 15 - 40℃; for 2h; |
Preparation of (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq) was slurried in toluene (10 vol). Vitride (2 eq) was added via addition funnel at a rate to maintain the temperature at 15-25 C. At the end of the addition, the temperature was increased to 40 C. for 2 hours (h), then 10% (w/w) aqueous (aq) NaOH (4.0 eq) was carefully added via addition funnel, maintaining the temperature at 40-50 C. After stirring for an additional 30 minutes (min), the layers were allowed to separate at 40 C. The organic phase was cooled to 20 C., then washed with water (2*1.5 vol), dried (Na2SO4), filtered, and concentrated to afford crude (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol that was used directly in the next step. |
|
With sodium bis(2-methoxyethoxy)aluminium dihydride; In toluene; at 15 - 40℃; for 2h; |
Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq)was slurried in toluene (l 0 vol). Vitride (2 eq) was added via addition funnel at a rate tomaintain the temperature at I 5-25 cc. At the end of the addition, the temperature was increasedto 40 oc for 2 hours (h), then 10% (w/w) aqueous (aq) NaOH (4.0 eq) was carefully added viaaddition funnel, maintaining the temperature at 40-50 oc. After stirring for an additional 30minutes (min), the layers were allowed to separate at 40 C. The organic phase was cooled to 20C, then washed with water (2 x 1.5 vol), dried (Na2S04), filtered, and concentrated to affordcrude (2,2-dit1uoro-1,3-benzodioxol-5-yl)-methanol that was used directly in the next step. |
|
|
Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq) is slurried in toluene (10 vol). Vitride (2 eq) is added via addition funnel at a rate to maintain the temperature at 15-25 C. At the end of addition the temperature is increased to 40 C for 2 h then 10% (w/w) aq. NaOH (4.0 eq) is carefully added via addition funnel maintaining the temperature at 40-50 C. After stirring for an additional 30 minutes, the layers are allowed to separate at 40 C. The organic phase is cooled to 20 C then washed with water (2 x 1.5 vol), dried (Na2SO4), filtered, and concentrated to afford crude (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol that is used directly in the next step. |
|
|
[00283] Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq) wasslurried in toluene (10 vol). Vitride (2 eq) was added via addition funnel at a rate to maintainthe temperature at 15-25 C. At the end of the addition, the temperature was increased to 40 ocfor 2 hours (h), then 10% (w/w) aqueous (aq) NaOH (4.0 eq) was carefully added via additionfunnel, maintaining the temperature at 40-50 C. After stirring for an additional30 minutes(min), the layers were allowed to separate at 40 C. The organic phase was cooled to 20 C, thenwashed with water (2 x 1.5 vol), dried (Na2S04), filtered, and concentrated to afford crude (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol that was used directly in the next step. |
|
|
Commercially available 2,2-difluoro-l,3-benzodioxole-5-carboxylic acid (1.0 eq) was slurried in toluene (10 vol). Vitride (2 eq) was added via addition funnel at a rate to maintain the temperature at 15-25 C. At the end of the addition, the temperature was increased to 40 C for 2 hours (h), then 10% (w/w) aqueous (aq) NaOH (4.0 eq) was carefully added via addition funnel, maintaining the temperature at 40-50 C. After stirring for an additional 30 minutes (min), the layers were allowed to separate at 40 C. The organic phase was cooled to 20 C, then washed with water (2 x 1.5 vol), dried (Na2S04), filtered, and concentrated to afford crude (2,2- difluoro-l ,3-benzodioxol-5-yl)-methanol that was used directly in the next step. |
|
With sodium bis(2-methoxyethoxy)aluminum hydride; In toluene; at 15 - 40℃; for 2h; |
Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq) was slurried in toluene (10 vol). Vitride (2 eq) was added via addition funnel at a rate to maintain the temperature at 15-25 C. At the end of the addition, the temperature was increased to 40 C. for 2 hours (h), then 10% (w/w) aqueous (aq) NaOH (4.0 eq) was carefully added via addition funnel, maintaining the temperature at 40-50 C. After stirring for an additional 30 minutes (min), the layers were allowed to separate at 40 C. The organic phase was cooled to 20 C., then washed with water (2*1.5 vol), dried (Na2SO4), filtered, and concentrated to afford crude (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol that was used directly in the next step. 5-Chloromethyl-2,2-difluoro-1,3-benzodioxole |
|
In toluene; |
Preparation of (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq) was slurried in toluene (10 vol). Vitride (2 eq) was added via addition funnel at a rate to maintain the temperature at 15-25 C. At the end of the addition, the temperature was increased to 40 C. for 2 hours (h), then 10% (w/w) aqueous (aq) NaOH (4.0 eq) was carefully added via addition funnel, maintaining the temperature at 40-50 C. After stirring for an additional 30 minutes (min), the layers were allowed to separate at 40 C. The organic phase was cooled to 20 C., then washed with water (2*1.5 vol), dried (Na2SO4), filtered, and concentrated to afford crude (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol that was used directly in the next step. |
|
With sodium bis(2-methoxyethoxy)aluminium dihydride; In toluene; at 15 - 40℃; for 2h; |
Preparation of (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq) was slurried in toluene (10 vol). Vitride (2 eq) was added via addition funnel at a rate to maintain the temperature at 15-25 C. At the end of the addition, the temperature was increased to 40 C. for 2 hours (h), then 10% (w/w) aqueous (aq) NaOH (4.0 eq) was carefully added via addition funnel, maintaining the temperature at 40-50 C. After stirring for an additional 30 minutes (min), the layers were allowed to separate at 40 C. The organic phase was cooled to 20 C., then washed with water (2*1.5 vol), dried (Na2SO4), filtered, and concentrated to afford crude (2,2-difluoro-1,3-benzodioxol-5-yl)-methanol that was used directly in the next step. |
|
|
Commercially available <strong>[656-46-2]2,2-difluoro-1,3-benzodioxole-5-carboxylic acid</strong> (1.0 eq.) was slurried in toluene (10 vol). Vitride (2 equivalents) was added via an addition funnel at a rate that maintained the temperature at 15-25 C. At the end of the addition, the temperature was raised to 40 C for 2 h, then 10% (w/w) aqueous NaOH (4.0 eq.) was carefully added via the addition funnel and the temperature was maintained at 40-50 C. After stirring for an additional 30 minutes (min), the layers were separated at 40 C. The organic phase was cooled to 20 C then washed with water (2 x 1.5 vol), dried (Na 2SO 4), filtered and concentrated to afford crude (2,2-difluoro-1,3-benzodioxol-5 -Base)-Methanol, which was used directly in the next step. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping