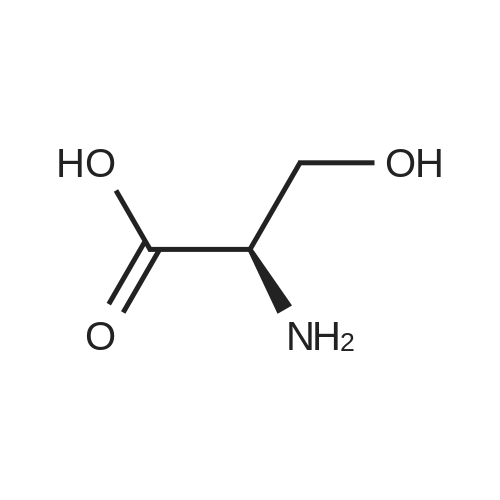
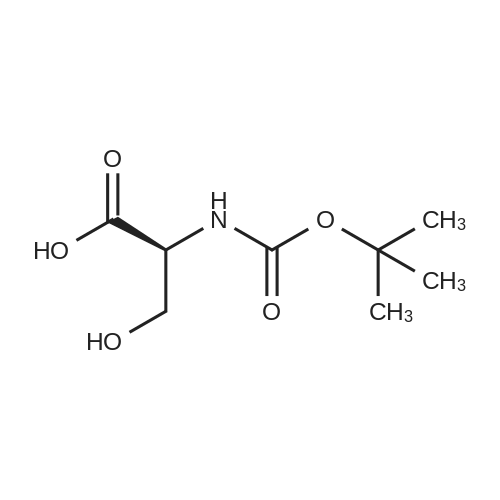
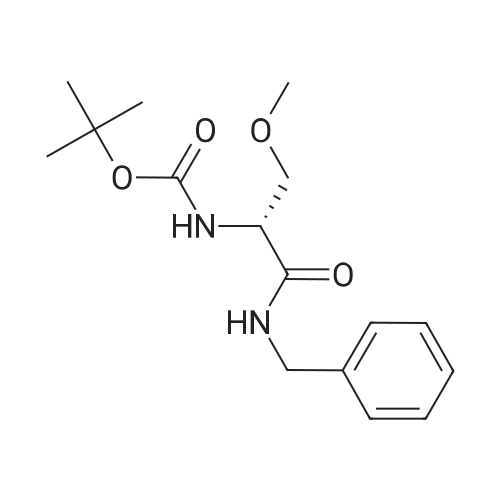
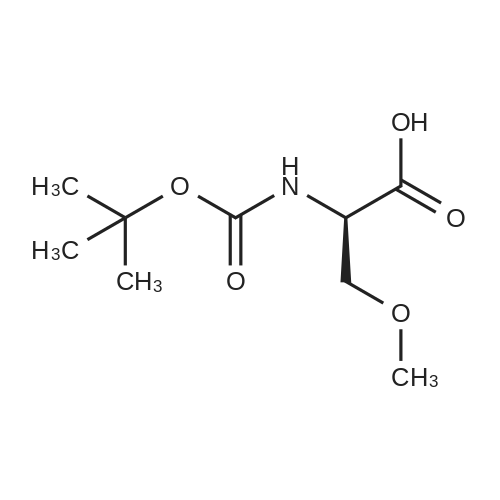
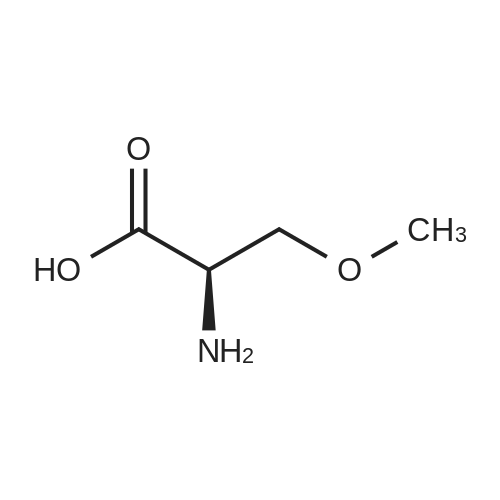
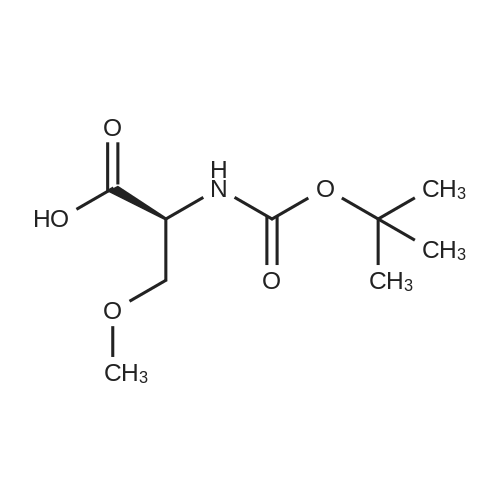
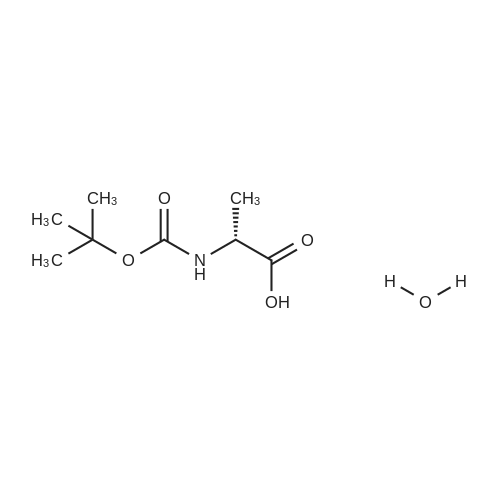
| 92.57% |
With sodium hydroxide; In water; at 20℃; for 14h; |
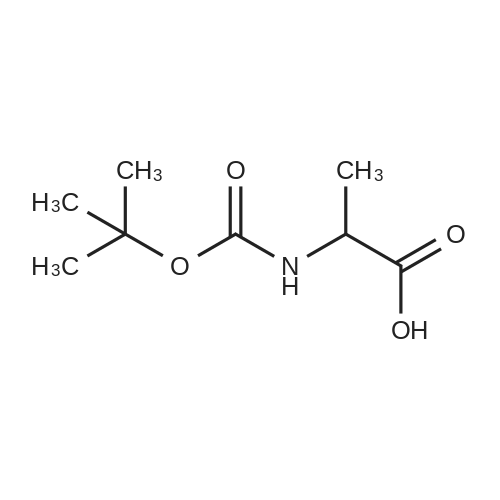
Water (5L) was charged at room temperature, sodium hydroxide (1.03 kg), D-serine (1 kg) with Di-tert-butyl dicarbonate (3.32 kg) into a reaction kettle, stir for 14 hours. Apply potassium bicarbonate solution 1 to adjust the pH to 2.5. filter, the filtrate was extracted with ethyl acetate, concentrated under reduced pressure, and toluene was charged to the reaction mass at 50 C and stirred at 45 C for 10 minutes. After cooling to room temperature, 0.01 kg stage I seed crystals were put in, stirred for 3 hours, centrifuged, and dried, to obtain lacosamide stage I. The yield was 92.57%. |
| 91% |
With triethylamine; In water; |
(A) Manufacture of (2R)-3-(3,5-bis(trifluoromethyl)benzyloxy)-2-tert-butoxycarbonylaminopropionic acid D-serine (25 g, 238 mmol) and triethylamine (35 mL, 250 mmol) were dissolved in water (400 mL), and Boc2O (50 g, 230 mmol) was added thereto and stirred at room temperature for 20 hours. The reaction mixture was washed with ethyl acetate (200 mL*2). The aqueous layer was acidified with 2 mol/L HCl to pH 2 and extracted with ethyl acetate (200 mL*5). The organic extract was dried over sodium sulfate anhydride. Sodium sulfate was filtered off and the solvent was removed by evaporation under reduced pressure to give Boc-D-Ser (44.4 g, 91%) as a colorless oil. |
| 91% |
With triethylamine; In water; |
Example 1. Manufacture of (2R)-3-{3,5-bis(trifluoromethyl)benzyloxy}-2-tert-butoxycarbonylaminopropionic acid D-serine (25 g, 238 mmol) and triethylamine (35 mL, 250 mmol) were dissolved in water (400 mL), and Boc2O (50 g, 230mmol) was added thereto and stirred at room temperature for 20 hours. The reaction mixture was washed with ethyl acetate (200 mL x 2). The aqueous layer was acidified with 2 mol/L HCl to pH 2 and extracted with ethyl acetate (200 mL x 5). The organic extract was dried over sodium sulfate anhydride. Sodium sulfate was filtered off and the solvent was removed by evaporation under reduced pressure to give Boc-D-Ser (44.4 g, 91%) as a colorless oil. |
| 85% |
With sodium hydroxide; In 1,4-dioxane; water; at 5 - 20℃; for 18h;pH 10; |
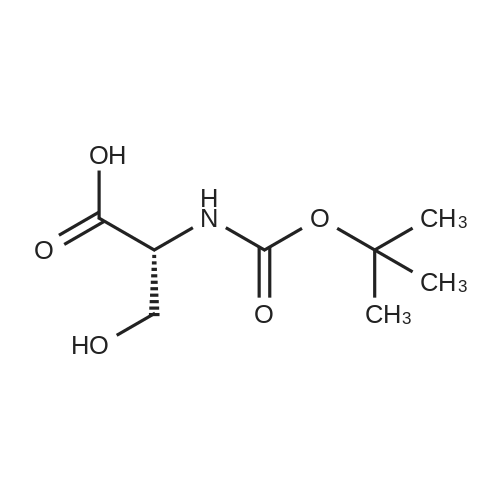
Intermediate 1: Preparation of (2R)-2-[(tert-butoxycarbonyl)amino]-3-hydroxypropanoic acidTo a cold solution (5 C.) of 74.4 g (1.86 mol) of sodium hydroxide in 1800 mL of water was added 95.1 g (0.90 mol) of D-serine. Then, a solution of 274.5 g (1.26 mol) of di-tert-butyldicarbonate (BOC2O) in 840 mL of 1,4-dioxane was added dropwise maintaining the temperature below 10 C. The reaction was stirred during 1 hour at 10 C. and for 2 hours at room temperature. The pH was adjusted to 10 with 50% sodium hydroxide (19.0 mL) and the reaction mixture was stirred at room temperature for 15 hours. Half of the reaction volume was evaporated under vacuum and after cooling the residue to 5 C. the pH was adjusted to 2-3 using 1N NaHSO4. The mixture was extracted with ethyl acetate (5×1000 mL) and the combined organic phase was dried over sodium sulphate and concentrated under vacuum. The resulting oil was dissolved in methyl tert-butyl ether (200 mL), petroleum oil (800 mL) was added and the mixture was stirred for 1 hour. The solid was collected by filtration and dried yielding 158.3 g of the title compound as a white solid (Yield 85%). |
| 53.2% |
With sodium hydroxide; In 1,4-dioxane; water; at 0 - 20℃; for 24h; |
General procedure: To a stirred solution of l-serine and d-serine (5.2g, 50.0mmol) in 1M NaOH aqueous solution (50mL) and 1, 4-dioxane (100mL), di-tert-butyl dicarbonate (13.1g, 60.0mmol) was slowly added at 0C. The mixture was warmed to room temperature and stirred for 24h. After evaporation of 1, 4-dioxane, the aqueous layer was wash with Et2O (50mL). The aqueous layer was acidified with 1M H2SO4 aqueous solution to give pH 2-3 and extracted with ethyl acetate (3×50mL). The combined organic layer was dried over anhydrous Na2SO4, filtrated, and concentrated under reduced pressure to give the product N-Boc-l-serine. |
|
With sodium hydroxide; In water; at 20 - 30℃; |
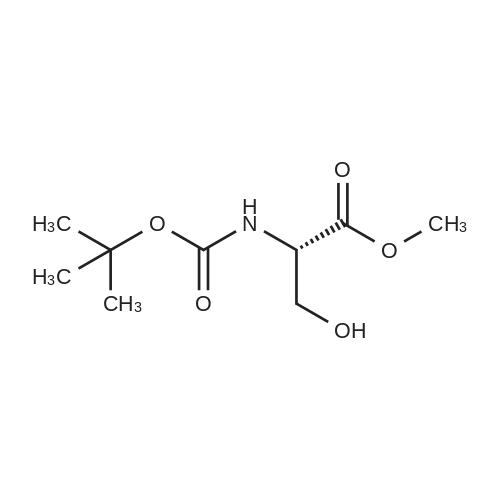
Example 1:Preparation of N-Boc-O-methyl-D-serine: Into a 2L, four-necked RB flask was charged 90 ml of water and 40 g of sodium hydroxide. <strong>[312-84-5]D-Serine</strong> is added to the solution at 20-25 C. Boc anhydride (150 g) is slowly added to the reaction mass keeping the temperature below 20C. The reaction mass was allowed to reach 25-30C and maintained for 16 h. TLC of the reaction mass showed the presence of D-serine content at <1.0% level. The reaction mass is cooled to 0-5C and started the simultaneous addition of aqueous sodium hydroxide (104 g of sodium hydroxide dissolved in 100 ml of water) and dimethyl sulphate (300 g) through addition funnels keeping the temperature below 5C. The reaction mass was maintained at same temperature till the completion of reaction. The reaction mass is diluted with water and extracted the product into diisopropyl ether. Aqueous layer is neutralized with citric acid to get < 3.5 pH. The reaction mass is extracted with diisopropyl ether and distilled of solvent to get 87 g of title compound as an oil. |
|
With sodium hydroxide; In water; at 20 - 25℃; for 10h; |
ExampIe-30: Preparation of N-Boc-D-serine of Formula 16:; Di-tert.butyldicarbonate (124.5 grams) was added to solution of D-serine (50 grams) in aqueous sodium hydroxide (38.5 g of NaOH in 250 ml of water) at 20-25C and stirred for 10 hours at 20-25C. The reaction mixture containing N-Boc-D-serine has been used directly to next stage with out any purification. |
|
|
To a stirred solution of sodium hydroxide 114 g (2.85 mole) in water (375 ml) was added (/?)-serine 250 g (2.37 mole). To the resulting clear solution of sodium salt of (i?)-serine were added t-butyloxycarbonic anhydride (Boc-anhydride), 571 g (2.61 mole) and a catalytic amount tetrabutylammonium bromidel2.0 g (0.036 mole). The mixture was stirred at ambient temperature for 16 hours. The resulting suspension was acidified to pH 3.5-4.0 with dilute HC1 (3N) and the product was extracted into dichloromethane to obtain a solution of (i?)-N-Boc-serine. |
|
With dmap; sodium hydroxide; In water; tert-butyl alcohol; at 20℃; for 11h; |
Preparation of N-Boc-D-serineSodium hydroxide (44 gm) was dissolved in water (300 ml) and stirred for 15 minutes. D-serine (105 gm) was added to the solution and then added dimethylaminopyridine (3 gm). Tert-butyl alcohol (180 gm) was added to the reaction mixture and then added di-tert-butyl dicarbonate (Boc anhydride, 230 gm) for 1 hour. The reaction mass was maintained for 10 hours at room temperature and then concentrated to obtain residual mass. The residual mass was then cooled to 5 to 10C and pH was adjusted to 2.9 with potassium bisulfate (IN). The reaction mass was extracted with ethyl acetate and the solvent was distilled off under vacuum at below 40C to obtain a residual solid of N-Boc-D-serine (190 gm). |
|
With sodium hydroxide; In water; at 20 - 30℃; for 16h; |
Into a 2 L, four-necked RB flask was charged 90 ml of water and 40 g of sodium hydroxide. <strong>[312-84-5]D-Serine</strong> is added to the solution at 20-25 C. Boc anhydride (150 g) is slowly added to the reaction mass keeping the temperature below 20 C. The reaction mass was allowed to reach 25-30 C. and maintained for 16 h. TLC of the reaction mass showed the presence of D-serine content at <1.0% level. The reaction mass is cooled to 0-5 C. and started the simultaneous addition of aqueous sodium hydroxide (104 g of sodium hydroxide dissolved in 100 ml of water) and dimethyl sulphate (300 g) through addition funnels keeping the temperature below 5 C. The reaction mass was maintained at same temperature till the completion of reaction. The reaction mass is diluted with water and extracted the product into diisopropyl ether. Aqueous layer is neutralized with citric acid to get <3.5 pH. The reaction mass is extracted with diisopropyl ether and distilled of solvent to get 87 g of title compound as an oil. |
|
|
To a stirred solution of sodium hydroxide 114 g (2.85 mole) in water (375 ml) was added <strong>[312-84-5](R)-serine</strong> 250 g (2.37 mole). To the resulting clear solution of sodium salt of <strong>[312-84-5](R)-serine</strong> were added t-butyloxycarbonic anhydride (Boc-anhydride) 571 g (2.61 mole) and a catalytic amount tetrabutylammonium bromide 12.0 g (0.036 mole). The mixture was stirred at ambient temperature for 16 hours. The resulting suspension was acidified to pH 3.5-4.0 with dilute HCl (3N) and the product was extracted into dichloromethane to obtain a solution of (R)-N-Boc-serine. |
|
With sodium hydroxide; In water; at 5 - 25℃; for 10.5h; |
To a stirred aqueous solution of sodium hydroxide (23.1 g of NaOH in 150 ml of water) was added D-serine (30 g) lot wise at 2G-25C and BOC anhydride (74.7 g) at 10-15C. The traction mixture was stirred at 20-25 0C for 10 hours. Sodium hydroxide pellets (11.4g) was added to the reaction mixture at 5-10C and stirred for another 30 mins. To the resulting reaction mixture was slowly added dimethylsulphate (340 ml) and aqueous solution of sodium hydroxide (91.0 g NaOH in 1 10 mL of water) at 5-10C over 4 hours and then stirred for 10 hrs. The reaction mixture was acidified to pH -1 -2 with 2% hydrochloric acid solution at 0-5C and extracted with methylene chloride (300 ml). Separating organic layer, aqueous layer was extracted with methylene chloride (100 mL). The combined organic layer was concentrated under vacuum. The obtained residue was dissolved in methyl tert-butyl ether (83.25 mL) at 60-65C. To the resulting solution was added H-hexane (466.2 mL) at 20-25C and mixture was stirred for 10-12h. The solid was filtered and dried to provide the title compound as white solid. Weight: 34.5 g Yield: 55.29%. |
|
With triethylamine; In methanol; at 20℃; |
To a stirring solution of (Boc)20 (1.09 g, 5 mmol) in CH3OH (5 mL) was added the suspension of (R)-2-amino-3-hydroxypropanoic acid SM 1 (525 mg, 5 mmol) and Et3N (507 mg, 5 mmol) in CH3OH (5 mL) at RT and stirred overnight. The reaction mixture was concentrated and the residue was diluted with H20. Adjusted the pH to 9-10 and extracted with DCM. The water layer was adjusted the pH to 2 with 4N HC1 aq. and extracted with DCM. Combined organic extracts were dried over anhydrous Na2SO4 and concentrated under reduced pressure to obtain crude compound 1 (950 mg, 92%) as a white solid. |
|
With sodium hydroxide; In water; at 50℃; |
To a solution of D-serine (150 g, 1.427 mol) and NaOH (68.5 g, 1.712 mol) in water (225 mL) was added di-tert-butyl dicarbonate (393.5 g, 1.712 mol) with stirring. The reaction mixture was heated to 50 C for 1-2 h (the initial clear reaction mass turns turbid in 1-2 h). The mixture was cooled to room temperature and diluted with of water (900 ml). The reaction mixture was washed with ethyl acetate (2 X 300 mL) to get rid of the traces of unreacted D-serine and other minor impurities. The pH of the reaction mixture was adjusted to 3-4 by slow addition of cold 3N HC1 (200 mL). The aqueous layer was extracted with ethyl acetate (3 X 400 mL). The combined organic layers of Boc-D-serine were washed with brine solution (300 mL) and used for the next stage. |
| 5 kg |
With sodium hydroxide; In tetrahydrofuran; water; at 0 - 20℃; for 18h;Large scale; |
Add 50L of water and 1.37kg of sodium hydroxide to the 50L reactor, and add 3kg of D-serine in batches. After the addition, cool down to 0-5 degrees, and dissolve 7kg of di-tert-butyl dicarbonate in 14L of tetrahydrofuran. Add the reaction solution, add room temperature to react for 18 hours, cool down to 0 C, add concentrated hydrochloric acid dropwise, adjust ph to 2-3.5, add 1.5 kg of ethyl acetate to extract three times, dry and spin dry to obtain 5 kg of colorless oil. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping