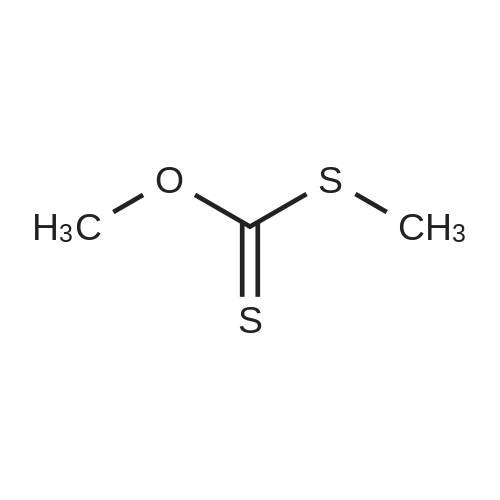
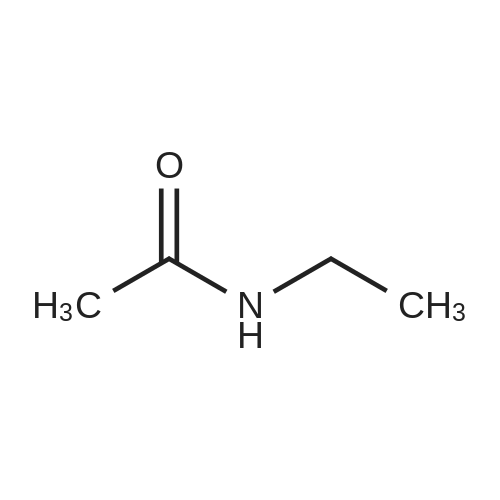
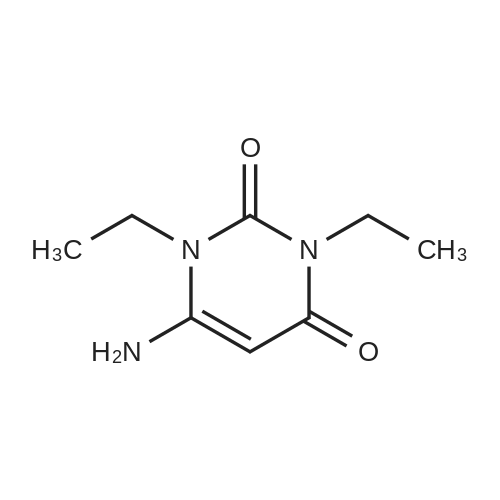
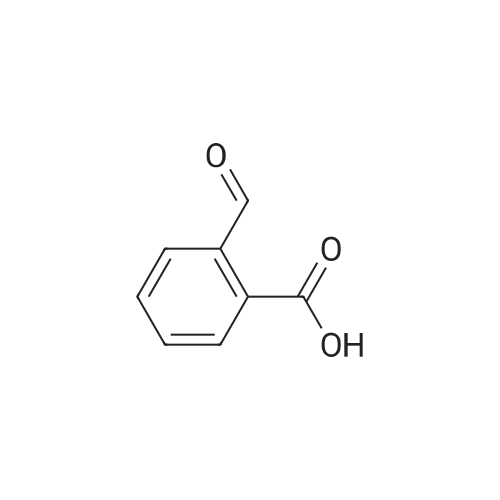
Alternatived Products of [ 623-76-7 ]
Product Details of [ 623-76-7 ]
CAS No. : 623-76-7
MDL No. : MFCD00009028
Formula :
C5 H12 N2 O
Boiling Point : -
Linear Structure Formula : -
InChI Key : ZWAVGZYKJNOTPX-UHFFFAOYSA-N
M.W :
116.16
Pubchem ID : 12194
Synonyms :
Calculated chemistry of [ 623-76-7 ]
Physicochemical Properties
Num. heavy atoms : 8
Num. arom. heavy atoms : 0
Fraction Csp3 : 0.8
Num. rotatable bonds : 4
Num. H-bond acceptors : 1.0
Num. H-bond donors : 2.0
Molar Refractivity : 32.34
TPSA : 41.13 Ų
Pharmacokinetics
GI absorption : High
BBB permeant : No
P-gp substrate : No
CYP1A2 inhibitor : No
CYP2C19 inhibitor : No
CYP2C9 inhibitor : No
CYP2D6 inhibitor : No
CYP3A4 inhibitor : No
Log Kp (skin permeation) : -6.94 cm/s
Lipophilicity
Log Po/w (iLOGP) : 1.37
Log Po/w (XLOGP3) : 0.1
Log Po/w (WLOGP) : 0.33
Log Po/w (MLOGP) : 0.42
Log Po/w (SILICOS-IT) : -0.3
Consensus Log Po/w : 0.38
Druglikeness
Lipinski : 0.0
Ghose : None
Veber : 0.0
Egan : 0.0
Muegge : 1.0
Bioavailability Score : 0.55
Water Solubility
Log S (ESOL) : -0.36
Solubility : 50.8 mg/ml ; 0.437 mol/l
Class : Very soluble
Log S (Ali) : -0.52
Solubility : 35.2 mg/ml ; 0.303 mol/l
Class : Very soluble
Log S (SILICOS-IT) : -1.46
Solubility : 4.01 mg/ml ; 0.0345 mol/l
Class : Soluble
Medicinal Chemistry
PAINS : 0.0 alert
Brenk : 0.0 alert
Leadlikeness : 1.0
Synthetic accessibility : 1.23
Application In Synthesis of [ 623-76-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
Downstream synthetic route of [ 623-76-7 ]
2
[ 22426-27-3 ]
[ 623-76-7 ]
[ 100401-73-8 ]
3
[ 623-76-7 ]
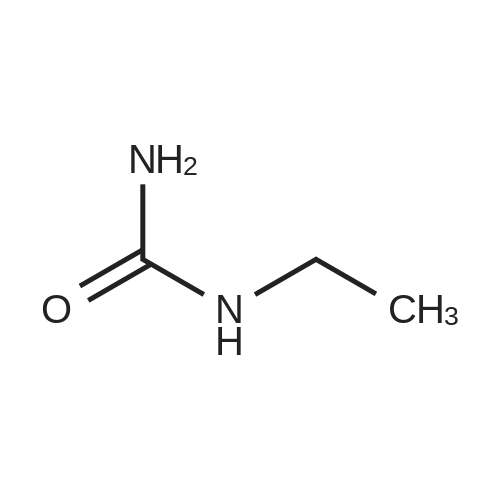
[ 44806-45-3 ]
[ 101082-94-4 ]
4
[ 7139-74-4 ]
[ 623-76-7 ]
5
[ 18804-23-4 ]
[ 623-76-7 ]
6
C8 H17 N3 O2
[ No CAS ]
[ 623-76-7 ]
[ 109-90-0 ]
7
[ 39938-75-5 ]
[ 623-76-7 ]
8
[ 871876-66-3 ]
[ 623-76-7 ]
[ 95745-38-3 ]
9
[ 623-76-7 ]
[ 1572-99-2 ]
[ 60664-11-1 ]
10
[ 868-84-8 ]
[ 75-04-7 ]
[ 623-76-7 ]
Reference:
[1]Synthesis,2007,p. 3497 - 3506
[2]Bulletin de la Societe Chimique de France,1910,vol. <4>7,p. 895,901
Annales de Chimie (Cachan, France),1912,vol. <8>25,p. 557
11
[ 19708-81-7 ]
[ 75-04-7 ]
[ 623-76-7 ]
12
[ 14467-74-4 ]
[ 75-04-7 ]
[ 623-76-7 ]
13
[ 75-04-7 ]
[ 109-90-0 ]
[ 623-76-7 ]
14
[ 623-76-7 ]
[ 64-19-7 ]
[ 625-50-3 ]
15
[ 623-76-7 ]
[ 372-09-8 ]
[ 41740-15-2 ]
Yield Reaction Conditions Operation in experiment
93.3%
In a 20 L four-necked flask equipped with nitrogen flow protection, oil bath, condenser and mechanical stirring and drying, 7000 mLAcetic anhydride was turned on, followed by 3400 g of 1,3-diethylurea and 2740 g of cyanoacetic acid. Oil bath slowly warmed to 75 ~ 85 , incubated for 2 hours. Liquid phase control showed that the raw material residue 1.6%. The reaction solution was concentrated to remove the solvent, and approximately 7000 mL of solvent was distilled off. 50L glass reactor into ice-water was cooled to 5-10 deg.] C, at temperature below 20 dropwise 34L has a good mass percent concentration with a 5% aqueous sodium hydroxide addition was complete and was cooled to 5 ~ 10 C for 1 hour. Filtered, the filter cake was washed with a small amount of ice water wet products, wet products in a forced air oven at 65 for 24 hours to obtain 5000g Yellow solid 6-amino-1,3-diethyl-1H-pyrimidine- Dione, purity: 95%; yield: 93.3%.
With acetic anhydride; at 78℃; for 2h;
1) 180 g 1,3-diethylformamide, 125 g cyanoacetic acid185ml acetic anhydride for ring-forming reaction,The reaction conditions were refluxed at 78 for 2h, the reaction was completed by adding sodium hydroxide, PH adjusted to neutral after filtration, washed three times, the resulting white solid is Compound ;
12.69 grams (0.14 mol) of diethylurea and 11.90 grams (0.14 mol) of cyanoacetic acid were heated in acetic anhydride at 60 0C for 3 hours, under dry atmosphere. Thereafter, the acetic anhydride and remaining cyanoacetic acid were evaporated, 5 % sodium hydroxide was added and the mixture was stirred and cooled. <n="68"/>20 grams of 6-amino~l,3-diethyluracil were obtained as a precipitate.
Reference:
[1]Patent: CN107141290,2017,A .Location in patent: Paragraph 0009; 0011
[2]Journal of Heterocyclic Chemistry,2017,vol. 54,p. 450 - 456
[3]Tetrahedron Letters,2005,vol. 46,p. 5727 - 5729
[4]Recueil des Travaux Chimiques des Pays-Bas,1946,vol. 65,p. 751,764
[5]Journal of the American Chemical Society,1953,vol. 75,p. 114
[6]Journal of Medicinal Chemistry,2006,vol. 49,p. 3682 - 3692
[7]Bioorganic and Medicinal Chemistry,2013,vol. 21,p. 7453 - 7464
[8]Patent: CN106496227,2017,A .Location in patent: Paragraph 0023; 0030; 0037
[9]Patent: WO2007/110868,2007,A2 .Location in patent: Page/Page column 66-67
[10]ChemMedChem,2020
16
[ 557-66-4 ]
[ 57-13-6 ]
[ 623-76-7 ]
Reference:
[1]Journal of the American Chemical Society,1923,vol. 45,p. 1820
Journal of the American Chemical Society,1929,vol. 51,p. 1799
[2]Journal of applied chemistry of the USSR,1982,vol. 55,p. 2467 - 2470
Zhurnal Prikladnoi Khimii (Sankt-Peterburg, Russian Federation),1982,vol. 55,p. 2715 - 2719
17
[ 623-76-7 ]
[ 119-67-5 ]
[ 107417-21-0 ]
18
[ 623-76-7 ]
[ 109-90-0 ]
[ 98487-66-2 ]
19
[ 109-90-0 ]
[ 623-76-7 ]
Reference:
[1]Synthetic Communications,2005,vol. 35,p. 1663 - 1674
[2]Journal of Organometallic Chemistry,1993,vol. 459,p. 131 - 138
[3]Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences,1851,vol. 32,p. 415
Justus Liebigs Annalen der Chemie,1851,vol. 80,p. 346
[4]Chemische Berichte,1869,vol. 2,p. 30
20
[ 693-29-8 ]
[ 623-76-7 ]
21
[ 22390-04-1 ]
[ 623-76-7 ]
22
[ 623-76-7 ]
[ 62516-93-2 ]
[ 62517-26-4 ]
23
[ 623-76-7 ]
[ 18522-92-4 ]
[ 1467-23-8 ]
24
[ 627-45-2 ]
[ 75-04-7 ]
[ 623-76-7 ]
25
[ 201230-82-2 ]
[ 75-04-7 ]
[ 623-76-7 ]
26
[ 623-76-7 ]
[ 1608-26-0 ]
[ 21243-32-3 ]
27
[ 50-00-0 ]
[ 623-76-7 ]
[ 6456-74-2 ]
[ 130750-07-1 ]
28
[ 50-00-0 ]
[ 623-76-7 ]
[ 3081-24-1 ]
[ 130750-10-6 ]
29
[ 50-00-0 ]
[ 623-76-7 ]
[ 26348-61-8 ]
[ 130767-32-7 ]
30
[ 13987-61-6 ]
[ 623-76-7 ]
[ 140367-28-8 ]
31
[ 4669-59-4 ]
[ 623-76-7 ]
[ 17882-69-8 ]
[ 18037-14-4 ]
2,2,6,6-Tetramethyl-3,5-diethyl-1-oxa-3,5-diaza-6-silacyclohexan-4-on
[ No CAS ]
1,3,5-Triethyl-2,2,4,4-tetramethyl-1,3,5-triaza-2,4-disilacyclohexan-6-on
[ No CAS ]
32
[ 4669-59-4 ]
[ 623-76-7 ]
[ 18037-14-4 ]
[ 98826-40-5 ]
2,2,6,6-Tetramethyl-3,5-diethyl-1-oxa-3,5-diaza-6-silacyclohexan-4-on
[ No CAS ]
1,3,5-Triethyl-2,2,4,4-tetramethyl-1,3,5-triaza-2,4-disilacyclohexan-6-on
[ No CAS ]
33
[ 623-76-7 ]
[ 10321-14-9 ]
[ 105480-47-5 ]
34
[ 623-76-7 ]
[ 18522-92-4 ]
[ 1467-23-8 ]
[ 75-04-7 ]
Reference:
[1]Journal of applied chemistry of the USSR,1982,vol. 55,p. 2467 - 2470
Zhurnal Prikladnoi Khimii (Sankt-Peterburg, Russian Federation),1982,vol. 55,p. 2715 - 2719
[2]Journal of applied chemistry of the USSR,1982,vol. 55,p. 2467 - 2470
Zhurnal Prikladnoi Khimii (Sankt-Peterburg, Russian Federation),1982,vol. 55,p. 2715 - 2719
35
[ 623-76-7 ]
[ 133611-28-6 ]
36
[ 623-76-7 ]
[ 85451-62-3 ]
37
[ 623-76-7 ]
[ 693-29-8 ]
Reference:
[1]Synthesis,1981,p. 373 - 374
[2]Bulletin de la Societe Chimique de France,1981,vol. <II> 2,p. 361 - 364
[3]Journal of the American Chemical Society,2007,vol. 129,p. 12690 - 12692
[4]Synthesis,1980,p. 755 - 757
38
[ 5710-11-2 ]
[ 623-76-7 ]
[ 27108-44-7 ]
[ 75-04-7 ]
39
[ 623-76-7 ]
[ 108-24-7 ]
[ 62898-99-1 ]
40
[ 75-44-5 ]
[ 75-04-7 ]
[ 623-76-7 ]
41
[ 42930-05-2 ]
[ 75-04-7 ]
[ 623-76-7 ]
42
[ 5710-11-2 ]
[ 75-04-7 ]
[ 623-76-7 ]
43
[ 201230-82-2 ]
[ 75-04-7 ]
[ 623-76-7 ]
[ 615-84-9 ]
44
[ 623-76-7 ]
[ 123-62-6 ]
[ 85102-61-0 ]
45
[ 623-76-7 ]
[ 111-30-8 ]
[ 86043-89-2 ]
46
[ 623-76-7 ]
[ 603-35-0 ]
[ 791-28-6 ]
[ 693-29-8 ]
47
C5 H11 N2 (15) NOS*H(1+)
[ No CAS ]
[ 623-76-7 ]
48
[ 109-90-0 ]
[ 623-76-7 ]
[ 101981-15-1 ]
49
[ 623-76-7 ]
[ 579-07-7 ]
[ 73633-54-2 ]
50
[ 623-76-7 ]
[ 513-85-9 ]
[ 144709-95-5 ]
51
[ 50-00-0 ]
[ 623-76-7 ]
[ 556-50-3 ]
[2-(3,5-Diethyl-4-oxo-[1,3,5]triazinan-1-yl)-acetylamino]-acetic acid
[ No CAS ]
52
[ 67-56-1 ]
[ 201230-82-2 ]
[ 75-04-7 ]
[ 6135-31-5 ]
[ 623-76-7 ]
53
[ 201230-82-2 ]
[ 75-04-7 ]
[ 6135-31-5 ]
[ 623-76-7 ]
54
[ 131543-46-9 ]
[ 623-76-7 ]
[ 53629-29-1 ]
55
[ 623-76-7 ]
[ 123-54-6 ]
[ 78994-41-9 ]
56
[ 623-76-7 ]
[ 201230-82-2 ]
[ 2043-61-0 ]
5-cyclohexyl-1,3-diethyl-imidazolidine-2,4-dione
[ No CAS ]
57
[ 201230-82-2 ]
[ 6667-19-2 ]
[ 623-76-7 ]
[ 14288-05-2 ]
[ 109-89-7 ]
diethylthiocarbamic acid S-phenyl ester
[ No CAS ]
58
[ 201230-82-2 ]
<i>N</i>,<i>N</i>-diethyl-<i>S</i>-(3-methoxy-phenyl)-thiohydroxylamine
[ No CAS ]
[ 623-76-7 ]
[ 14288-05-2 ]
[ 109-89-7 ]
diethyl-thiocarbamic acid <i>S</i>-(3-methoxy-phenyl) ester
[ No CAS ]
59
[ 623-76-7 ]
cyano-cyclopentyl-acetic acid alkyl ester
[ No CAS ]
[ 100530-21-0 ]
60
[ 623-76-7 ]
cyano-cyclohexyl-acetic acid alkyl ester
[ No CAS ]
[ 100967-97-3 ]
61
[ 623-76-7 ]
cyano-cycloheptyl-acetic acid alkyl ester
[ No CAS ]
[ 101087-06-3 ]
Yield Reaction Conditions Operation in experiment
Among the compounds of formula (I), mention may be made especially of the following preferred compounds: urea ... N,N-diethylurea N,N-dipropylurea 1-cyclopentyl-1-methylurea 1,3-dimethylurea 1,3-diethylurea 1,3-bis(2-hydroxethyl)urea 1,3,bis(2-hydroxypropyl)urea 1,3-bis(3-hydroxypropyl)urea ...
Among the compounds of formula (I), mention may be made especially of the following particularly preferred compounds: urea ... diallylurea 2-chloroethylurea N,N-dimethylurea 1,3-dimethylurea 1,3-diethylurea 1,3-bis(2-hydroxyethyl)urea 1,3-dipropylurea 1-ethyl-3-propylurea ...
68
[ 124-38-9 ]
[ 75-04-7 ]
[ 623-76-7 ]
69
[ 75-04-7 ]
carbon monoxide
[ No CAS ]
[ 623-76-7 ]
70
[ 75-04-7 ]
diguaiacyl carbonate
[ No CAS ]
[ 623-76-7 ]
71
N-ethyl-carbamate ethylamine
[ No CAS ]
[ 623-76-7 ]
72
[ 623-76-7 ]
[ 7732-18-5 ]
[ 7782-50-5 ]
[ 872822-82-7 ]
73
[ 623-76-7 ]
[ 7782-77-6 ]
[ 49540-32-1 ]
74
[ 7647-01-0 ]
[ 623-76-7 ]
nitrogen
[ No CAS ]
[ 557-66-4 ]
[ 109-90-0 ]
75
[ 623-76-7 ]
[ 15909-81-6 ]
76
[ 623-76-7 ]
[ 1079-66-9 ]
[ 390810-98-7 ]
77
[ 623-76-7 ]
[ 485-47-2 ]
1,3,3a,8a-tetrahydro-3a,8a-dihydroxy-1,3-diethylindeno[1,2-d]imidazole-2,8-dione
[ No CAS ]
78
[ 75-04-7 ]
[ 57-13-6 ]
[ 623-76-7 ]
79
[ 292638-84-7 ]
[ 623-76-7 ]
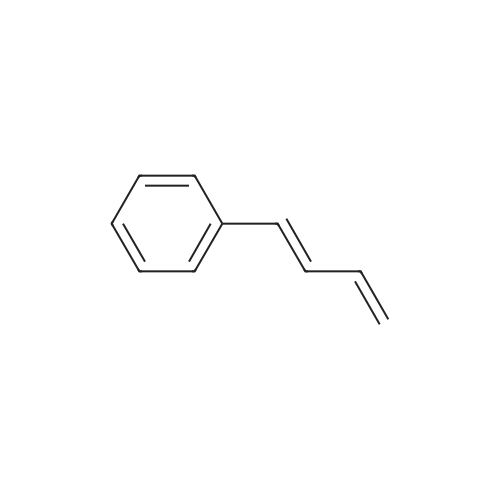
1,3-Diethyl-1-((E)-styryl)-urea
[ No CAS ]
80
[ 20237-34-7 ]
[ 623-76-7 ]
4-((E)-But-1-enyl)-1,3-diethyl-imidazolidin-2-one
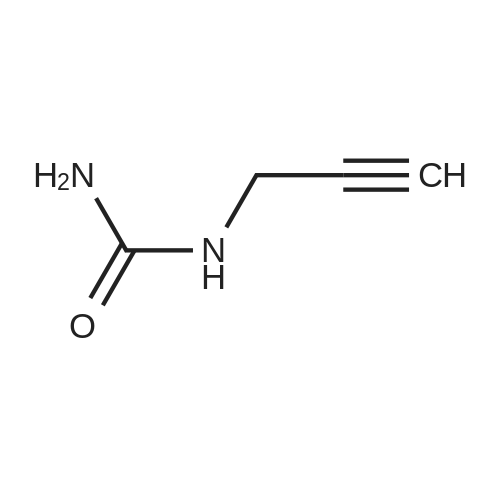
[ No CAS ]
81
[ 131543-46-9 ]
[ 625-52-5 ]
[ 623-76-7 ]
[ 263403-93-6 ]
82
[ 623-76-7 ]
[ 2787-45-3 ]
1,3-Diethyl-4-((Z)-1-methyl-propenyl)-imidazolidin-2-one
[ No CAS ]
83
[ 623-76-7 ]
[ 876938-53-3 ]
(3aS,8aR)-1,3-Diethyl-3,3a,4,5,6,8a-hexahydro-1H-cycloheptaimidazol-2-one
[ No CAS ]
84
[ 623-76-7 ]
[ 16939-57-4 ]
1,3-Diethyl-4-((E)-styryl)-imidazolidin-2-one
[ No CAS ]
85
[ 623-76-7 ]
[ 32507-39-4 ]
1,3-Diethyl-4-[(E)-2-(4-methoxy-phenyl)-vinyl]-imidazolidin-2-one
[ No CAS ]
86
[ 623-76-7 ]
[ 4165-81-5 ]
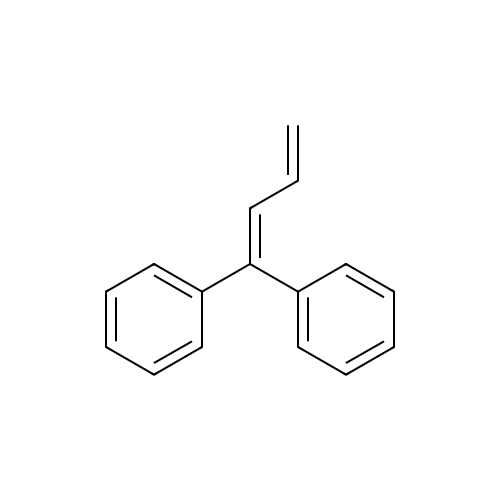
4-(2,2-diphenyl-vinyl)-1,3-diethyl-imidazolidin-2-one
[ No CAS ]
87
[ 623-76-7 ]
[ 123751-89-3 ]
1,3-Diethyl-4-((E)-2-naphthalen-2-yl-vinyl)-imidazolidin-2-one
[ No CAS ]
88
[ 623-76-7 ]
[ 55177-38-3 ]
1,3-Diethyl-4-((E)-2-phenyl-propenyl)-imidazolidin-2-one
[ No CAS ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping