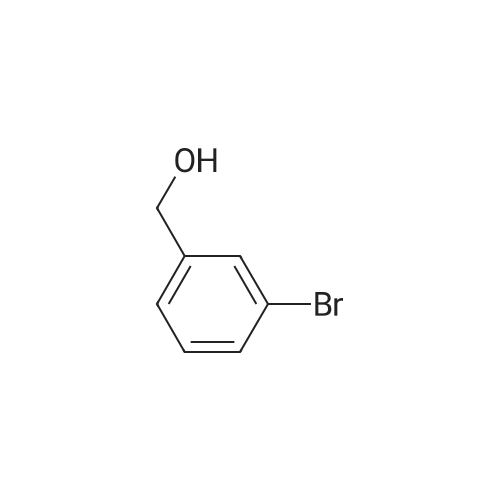
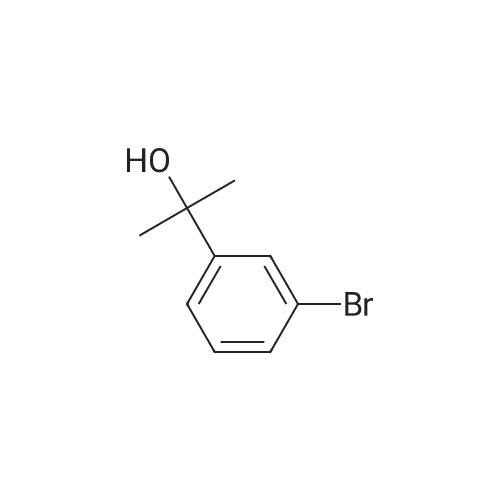
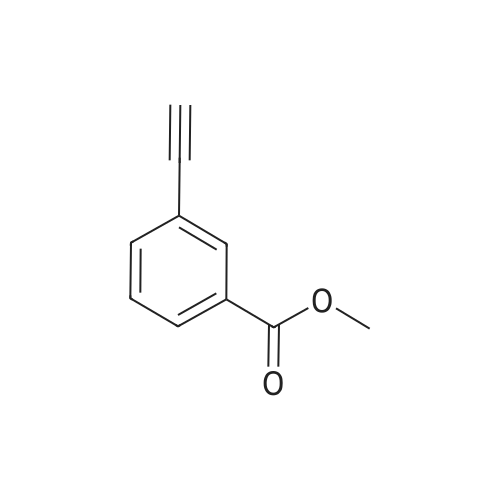
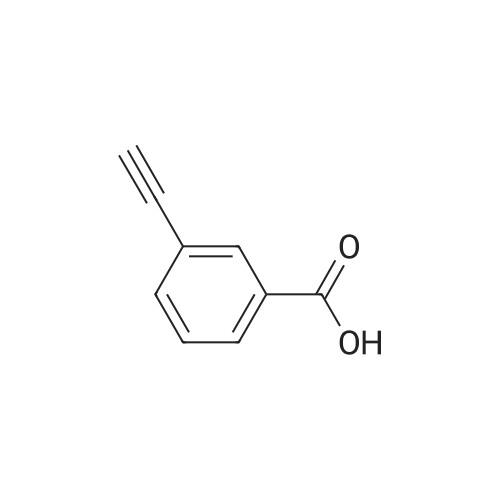
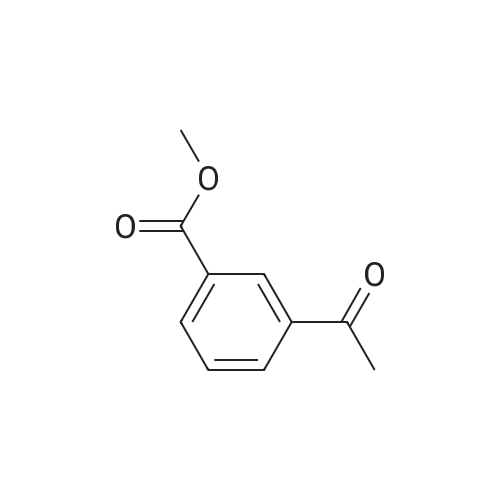
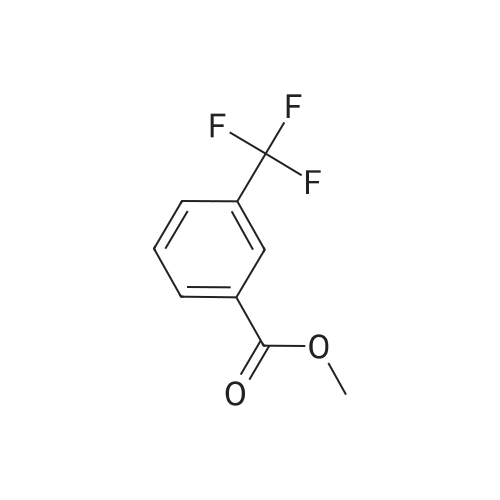
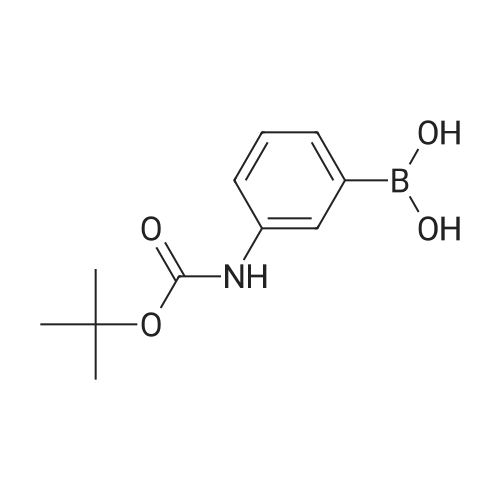
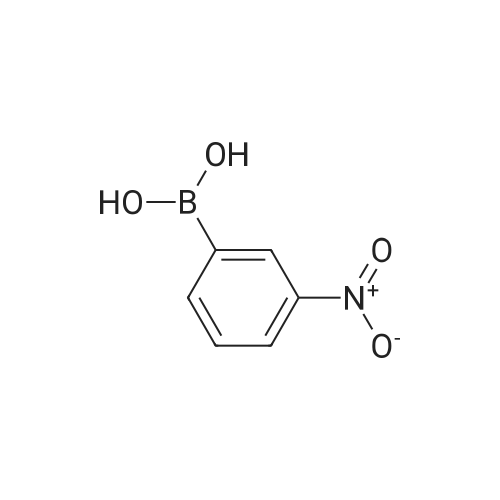
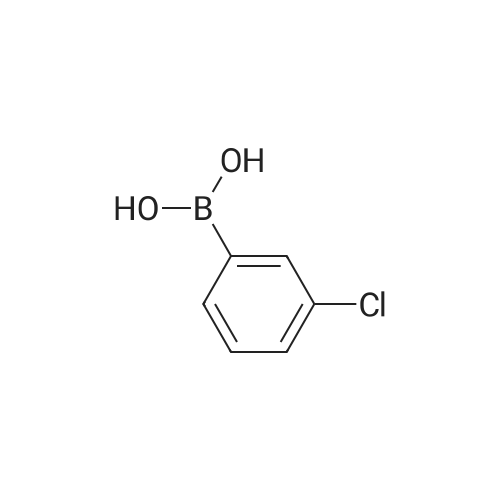
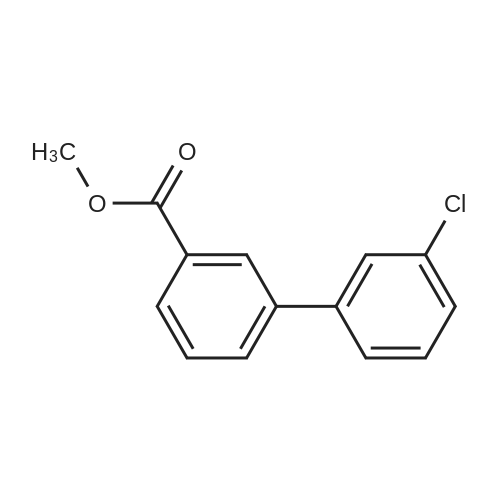
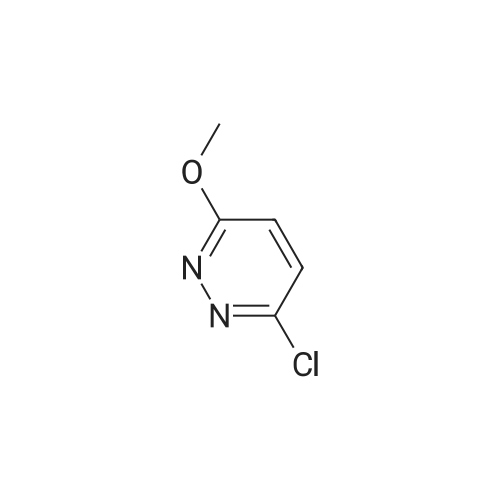
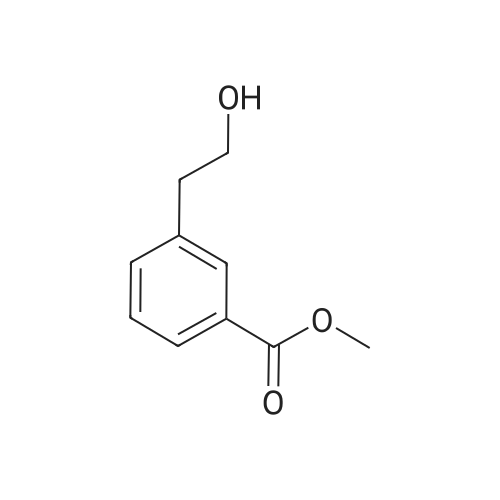
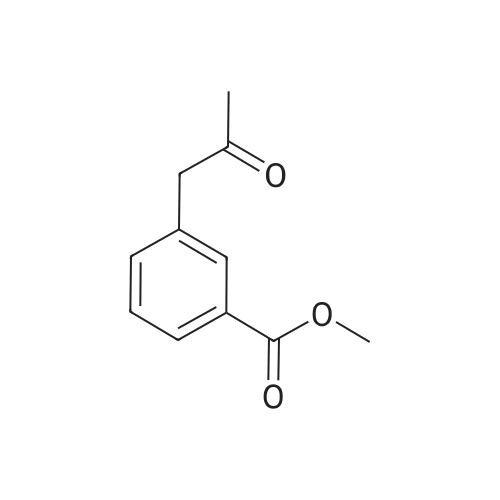
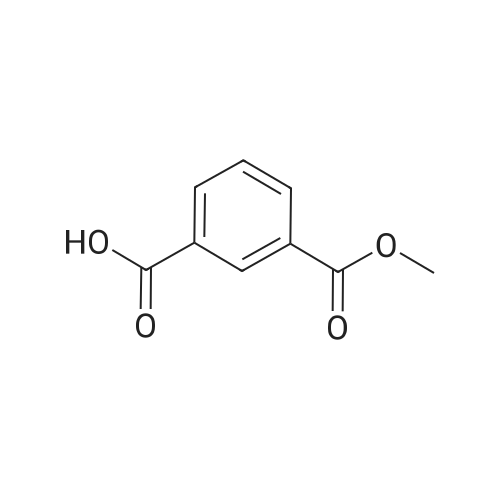
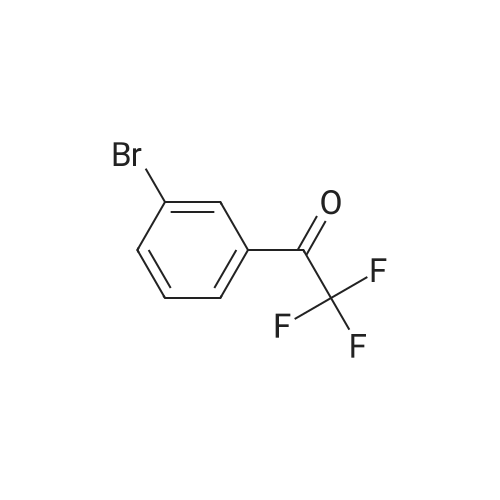
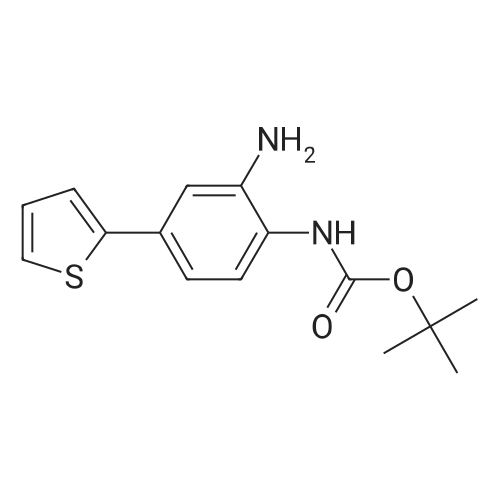
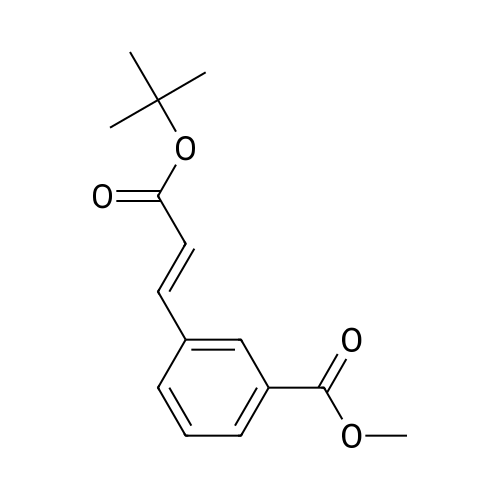
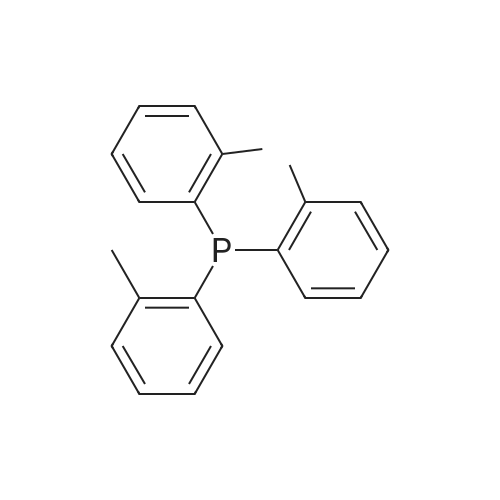
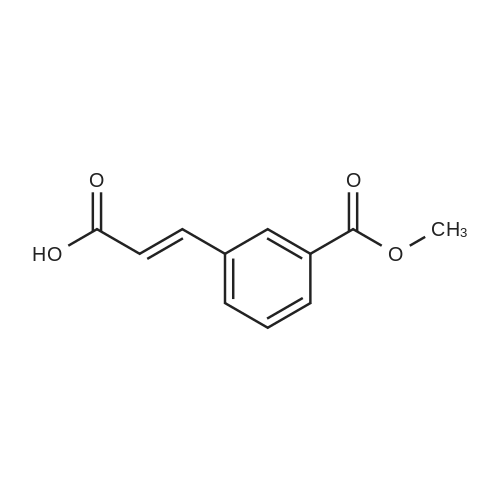
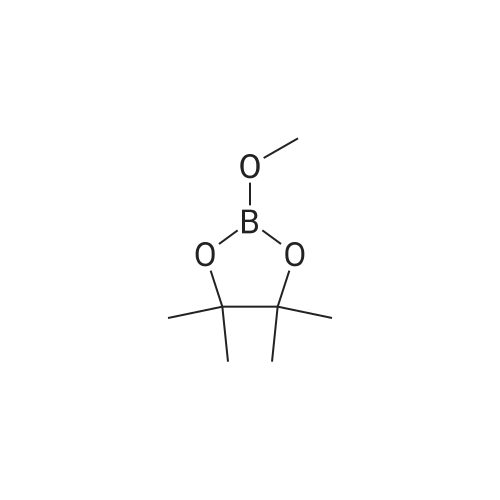
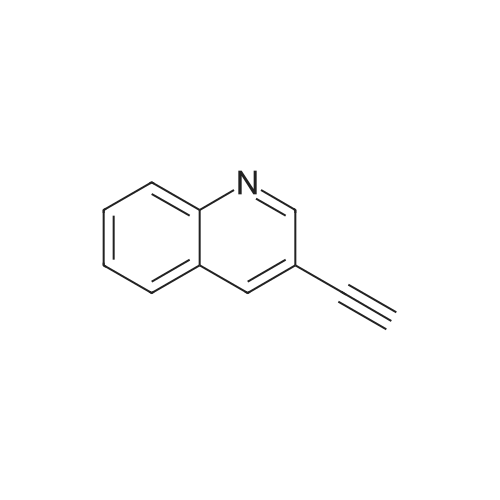
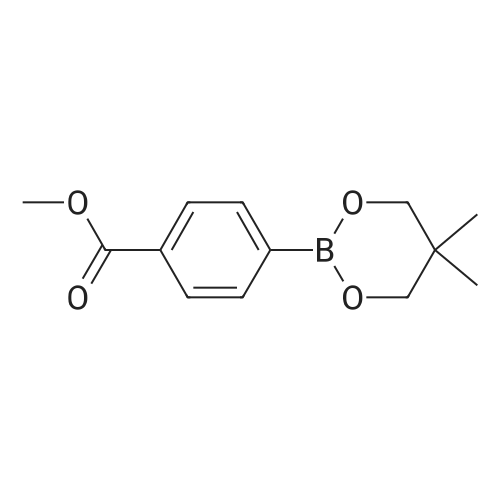
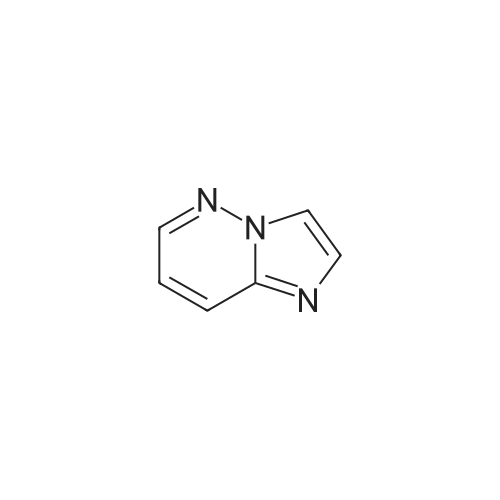
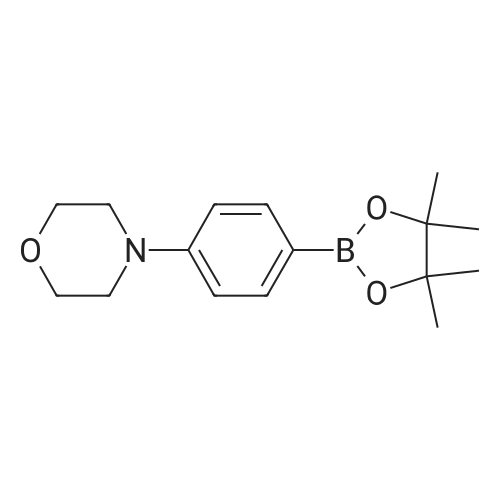
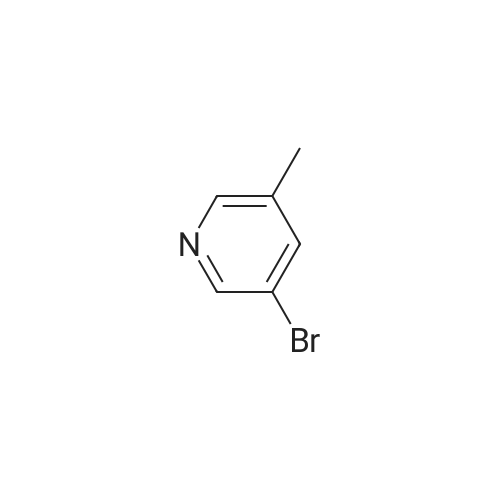
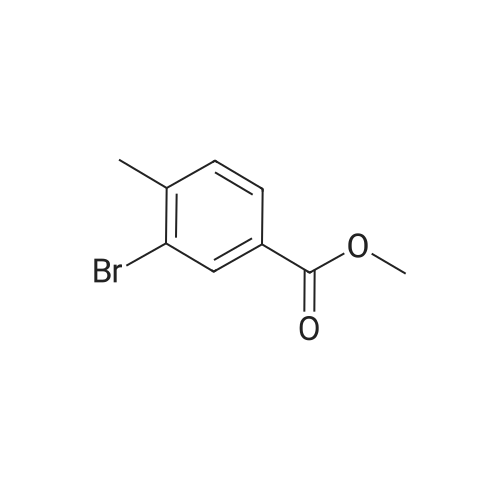
| 85% |
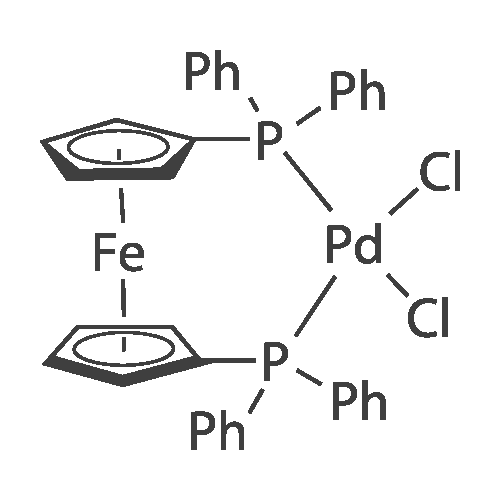
With palladium diacetate; caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; In toluene; at 100℃; for 12h;Inert atmosphere; |
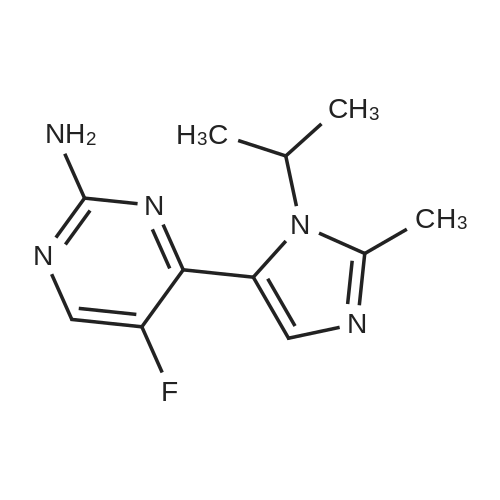
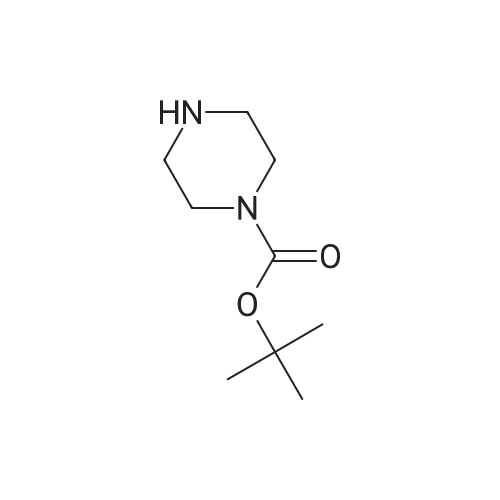
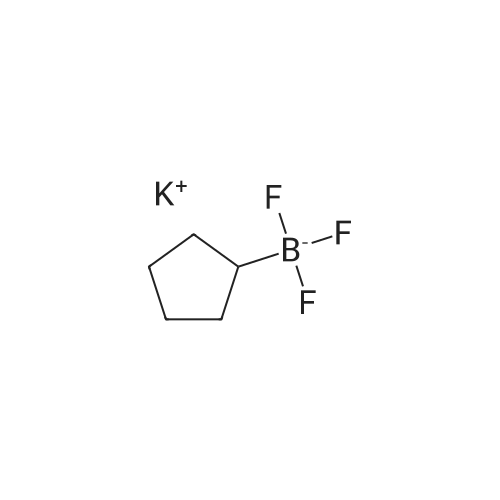
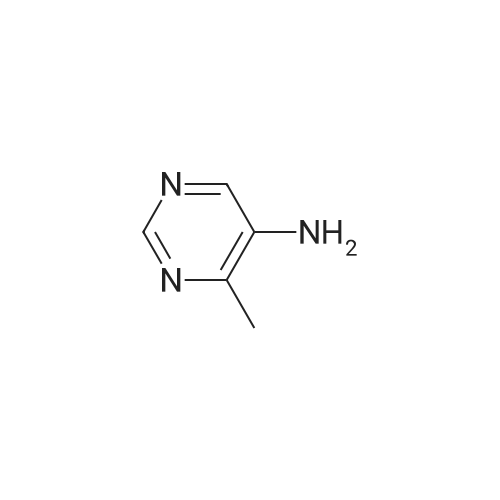
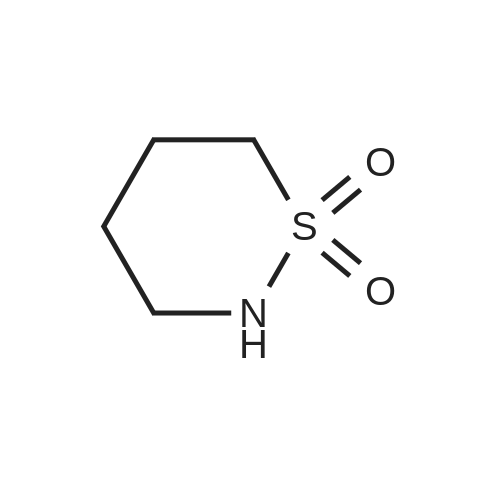
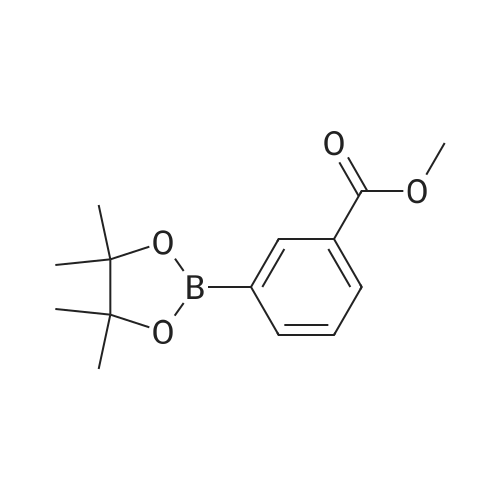
A mixture of 86 (3.00 g, 14.0 mmol), /V-Boc-piperazine (2.73 g, 14.7 mmol), Pd(OAc)2(313 mg, 1.40 mmol), Cs2C03(9.09 g, 27.9 mmol) and BINAP (869 mg, 1.40 mmol) in toluene (20 mL) was degassed and purged with N2for 3 times, and then the mixture was stirred at 100 C for 12 h under N2atmosphere. The reaction mixture was filtered and concentrated under reduced pressure to give 87 (3.80 g, 85%) as a pale yellow solid.1H N R (400 MHz, CDCI3) 7.60 (s, 1 H), 7.55 (d, J = 7.6 Hz, 1 H),7.33 (t, J - 8.0 Hz, 1 H), 7.13 (d, J - 2.4 Hz, 1 H), 3.91 (s, 3H), 3.60 (t, J = 4.4 Hz, 4H), 3.19 (t, J = 4.8 Hz, 4H), 1 .49 (s, 9H). To a solution of 87 (1 .00 g, 3.12 mmol) in THF (20 mL) was added LiAIH4(1 18 mg,3.12 mmol). The mixture was stirred at 0 C for 1 h. The reaction mixture was quenched by sodium potassium tartrate (0.5 mL) at 15 C, and then filtered and concentrated under reduced pressure to give 88 (700 mg, 77%) as a pale yellow oil.1H NMR (400 MHz, CDCI3) 7.28-7.25 (m, 1 H), 6.96 (s, 1 H), 6.89-6.84 (m, 2H), 4.66 (s, 2H), 3.58 (t, J = 4.8 Hz, 4H), 3.15 (t, J = 4.8 Hz, 4H), 1.49 (s, 9H). To a solution of 88 (293 mg, 1.00 mmol) and Et3N (304 mg, 3.00 mmol) in DCM (10 mL) was added MsCI (229 mg, 2.00 mmol). The mixture was stirred at 0 C for 2 h. The reaction mixture was quenched by addition H20 (20 mL) at 15 C, and then extracted with EtOAc (20 mL x 2). The combined organic layers were washed with brine (10 mL x 2), dried over Na2S04, filtered and concentrated under reduced pressure to give crude 89 (200 mg, yellow solid) which was used in the next step without further purification. MS (ESI): mass calcd. for C17H26N205S 370.16, m/z found 371.2 [M+H]+. A mixture of 89 (300 mg, 810 umol), 6-003 (236 mg, 0.810 mmol) and K2C03(336 mg, 2.43 mmol) in DMF (5 mL) was stirred at 60 C for 1 h under N2atmosphere. The reaction mixture was quenched by H20 (10 mL) at 15 C, and then extracted with EtOAc (20 mL x 2). The combined organic layers were washed with brine (10 mL x 2), dried over Na2S04, filtered and concentrated under reduced pressure to give crude 90 (400 mg, pale yellow oil) which was used in the next step without further purification. MS (ESI): mass calcd. for C3oH4oBN307565.30, m/z found 566.3 [M+H]+. To a solution of 90 (400 mg, 0.707 mmol) in EtOAc (10 mL) was added HCI/EtOAc (6 M, 1.18 mL). The mixture was stirred at 15 C for 1 h. After concentrated under reduced pressure, the reaction mixture was purified by prep. HPLC (column: Luna C18 100 x 30 mm; liquid phase: [A-HCI/H2O=0.040% v/v; B-ACN] B%: 13%-43%, 12 min]) to give 6-126 (102 mg, 28%) as a white solid.1H NMR (400 MHz, DMSO-d6) 9.17 (s, 2H), 8.58 (d, J = 7.2 Hz, 1 H),7.34 (d, J = 7.2 Hz, 1 H), 7.28-7.22 (m, 2H), 7.01 (s, 1 H), 6.95 (d, J = 8.0 Hz, 1 H), 6.88 (d, J = 7.2 Hz, 1 H), 5.13 (s, 2H), 4.97 (s, 2H), 4.34 (t, J = 7.2 Hz, 1 H), 3.36 (d, J = 5.2 Hz, 4H), 3.17 (d, J = 2.8 Hz, 4H), 2.44 (s, 3H), 2.20-2.11 (m, 6.8 Hz, 1 H), 0.958-0.942 (m, 6 H); ESI-MS m/z 466 [M+H]+; HPLC purity: 97.27% (220 nm), 96.92% (254 nm) |
| 30% |
With palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate; In toluene; at 100℃; for 0.25h;Inert atmosphere; |
A mixture of tert-butyl piperazine-1-carboxylate (1.0 eq), 17 (1.0 eq.), BINAP (5% mol) and sodium tert-butoxide (3.0 eq) were placed in two neck round bottom flask. Then anhydrous toluene was added to the mixture. The mixture was degassed and back-filled with dry nitrogen gas before suspension of palladium (II) acetate (5%mol) in dry toluene was added. The mixture reaction was stirred at 100 oC for 15min, cooled to room temperature, quenched by water. Normal extraction and purification was applied to get white solid, yield 30%.1H NMR (300MHz, CDCl3) δ 7.59 (s, 1H), 7.54 (d, J = 7.68 Hz, 1H), 7.33 (t, J = 8.04 Hz, 1H), 7.10 (d, J = 8.25 Hz, 1H), 3.91 (s, 3H), 3.59 (t, J = 4.95 Hz, 4H), 3.18 (t, J = 5.31 Hz, 4H), 1.49 (s, 9H). |
| 23% |
With palladium diacetate; caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; In 1,4-dioxane; for 18h;Inert atmosphere; Reflux; |
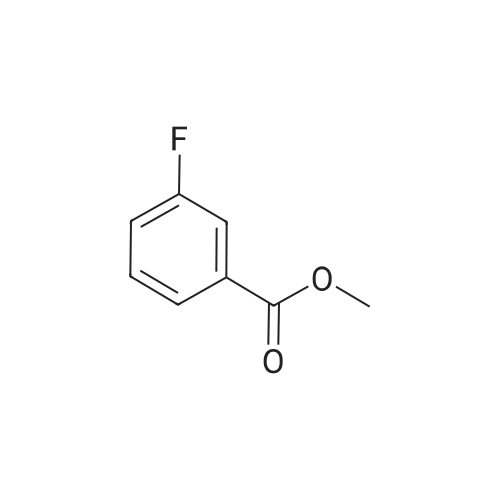
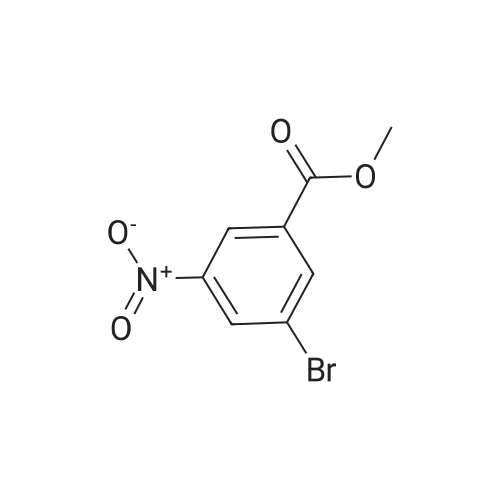
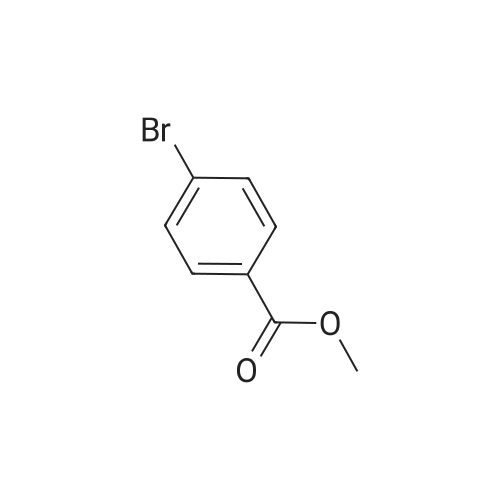
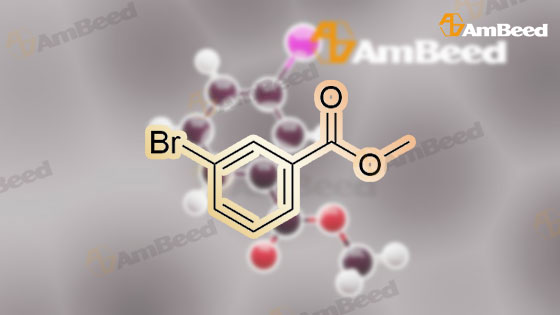
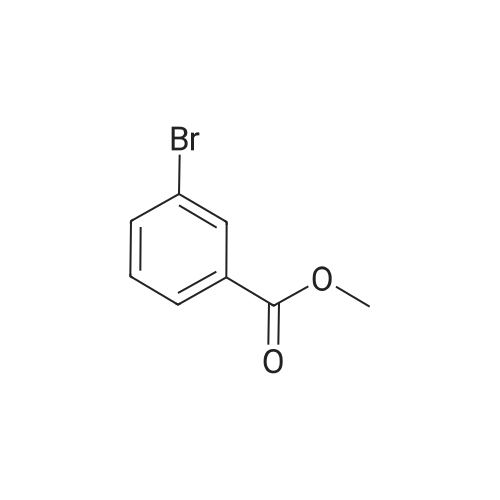
To a 250 mL round-bottom flask containing 40 mL of 1,4-dioxane, raw material 2-23 (<strong>[618-89-3]methyl m-bromobenzoate</strong>) (2.15 g, 10 mmol), BINAP (622 mg, 1 mmol), palladium acetate (45 mg, 0.2 mmol), cesium carbonate (6.5 g, 20 mmol), Boc-piperazine 2-24 (1.86 g, 10 mmol) were added in sequence, and reacted under argon atmosphere and reflux at 100 C for 18 hours, until TLC (PE: EA = 10: 1) detection showed the reaction was completed. Filtration was carried out with diatomite to remove solid(s). The filtrate was evaporated under reduced pressure and subjected to column chromatography (PE: EA = 10: 1) to give 720 mg of a light yellow oily product, yield: 23%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping