| 28% |
With water; potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In 1,2-dimethoxyethane; at 150℃; for 0.25h; |
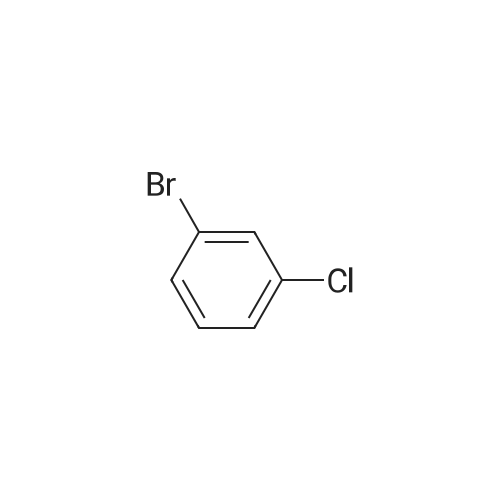
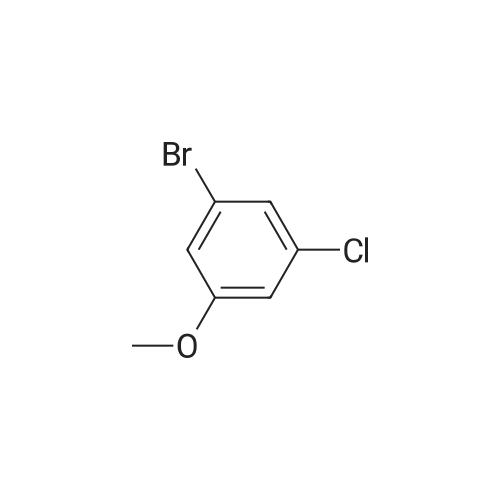
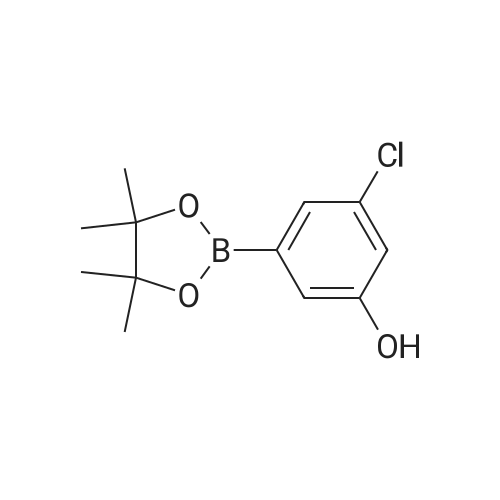
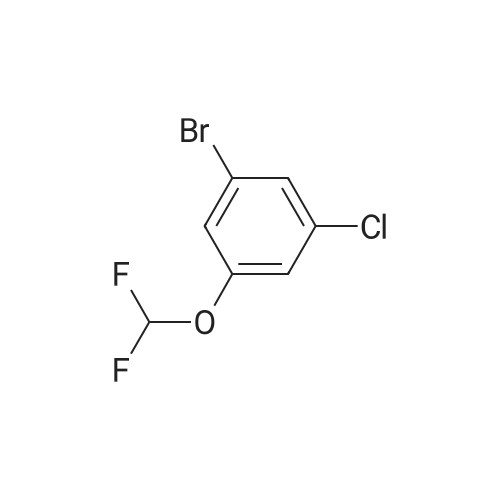
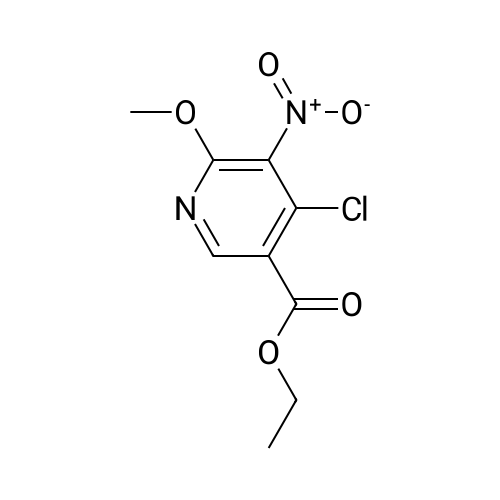
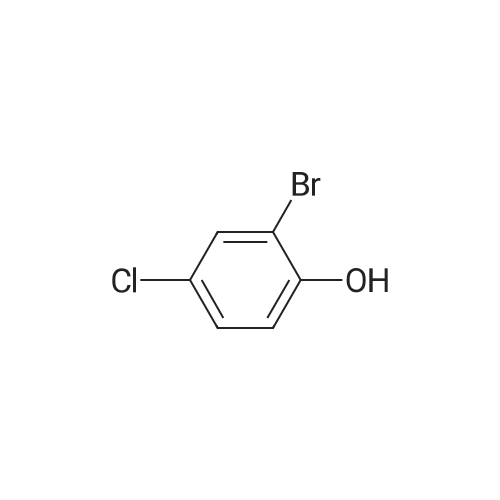
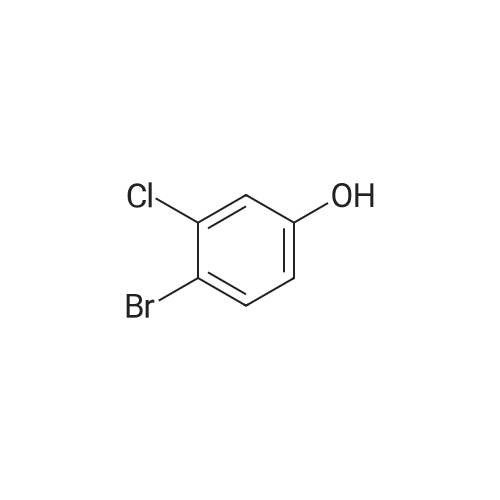
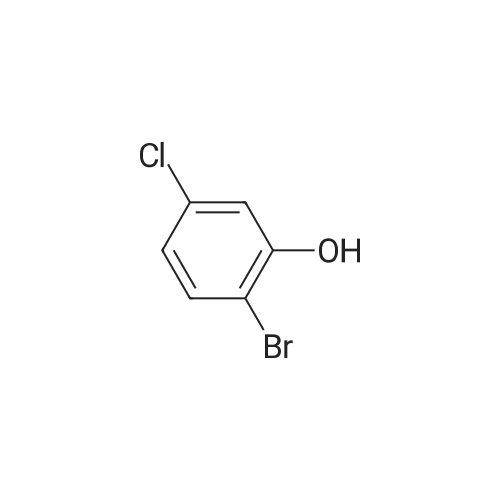
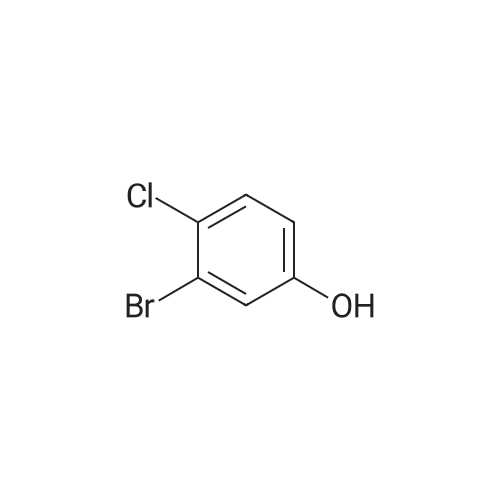
<strong>[56962-04-0]3-Bromo-5-chlorophenol</strong> (5 g, 19.9 mmol, described in: Maleczka R. E. et. al. J. Am. Chem. Soc. 2003, 125, 7792-7793), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi-1,3,2-dioxaborolane (6.06 g, 23.9 mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium(II) chloride dichloromethane adduct (487 mg, 0.6 mmol), potassium acetate (5.86 g, 59.7 mmol), 1,2-dimethoxyethane (60 mL) and water (4 mL) were divided into four microwave vials and irradiated in a microwave at 150° C. for 15 min each. When cooled to ambient temperature the mixtures were pooled, diluted with brine and extracted with diethyl ether. The combined organic phases were dried over sodium sulfate and concentrated in vacuo. Purified by column chromatography, using a gradient with 0-5percent acetonitrile in dichloromethane as the eluent, to give 1.43 g (28percent yield) of the title compound: 1H NMR (DMSO-d6) delta 9.89 (s, 1H), 7.02 (s, 2H), 6.91 (s, 1H), 1.28 (s, 12H); MS (ES) m/z 253 [M-H]-. |
| 28% |
With potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In 1,2-dimethoxyethane; water; at 150℃; for 0.25h;Irradiation in a microwave; |
<strong>[56962-04-0]3-Bromo-5-chlorophenol</strong> (5 g, 19.9 mmol, described in: Maleczka R. E. et. al. J. lambda(roe. Chem. Soc. 2003, 725, 7792-7793), 454,4',4',5,5,5l,5I-octamethyl-2,2'-bi-l>3,2- dioxaborolane (6.06 g, 23.9 mmol), [l,r-bis(diphenylphosphino)ferrocene]palladium(II) chloride dichloromethane adduct (487 mg, 0.6 mmol), potassium acetate (5.86 g, 59.7 mmol), 1,2-dimethoxyethane (60 mL) and water (4 mL) were divided into four microwave vials and irradiated in a microwave at 150 °C for 15 min each. When cooled to room temperature the mixtures were pooled, diluted with brine and extracted with diethyl ether. The combined organics were dried over sodium sulfate and concentrated in vacuo. Purified by column chromatography, using dichloromethane/acetonitrile (95:5) as the eluent, to give 1.43 g (28percent yield) of the title compound: 1H NMR (DMSO-^6) delta 9.89 (s, 1 H) 7.02 (s, 2 H) 6.91 (s, 1 H) 1.28 (s, 12 H); MS (ESI) m/z 253 [M-H]\\ |
| 28% |
With potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In 1,2-dimethoxyethane; water; at 150℃; for 0.25h;Microwave irradiation; |
Example 5 3-Chloro-5-(4A5,5-tetramethyl-1.3.2-dioxaborolan-2-yl)phenol; <strong>[56962-04-0]3-Bromo-5-chlorophenol</strong> (5 g, 19.9 mmol, described in: Maleczka R. E. et. al. J. Am. Chem. Soc. 2003, 125, 7792-7793), 4,4,4',4',5,5,51,51-octamethyl-2,2'-bi-l,3,2- dioxaborolane (6.06 g, 23.9 mmol), [l,r-bis(diphenylphosphino)ferrocene]palladium(II) chloride dichloromethane adduct (487 mg, 0.6 mmol), potassium acetate (5.86 g, 59.7 mmol), 1 ,2-dimethoxyethane (60 mL) and water (4 mL) were divided into four microwave <n="50"/>vials and irradiated in a microwave at 150 °C for 15 min each. When cooled to ambient temperature the mixtures were pooled, diluted with brine and extracted with diethyl ether. The combined organic phases were dried over sodium sulfate and concentrated in vacuo. Purified by column chromatography, using a gradient with 0-5percent acetonitrile in dichloromethane as the eluent, to give 1.43 g (28percent yield) of the title compound: 1H NMR (DMSOd6) delta 9.89 (s, 1 H), 7.02 (s, 2 H), 6.91 (s, 1 H), 1.28 (s, 12 H); MS (ES) m/z 253 [M-I]-. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping