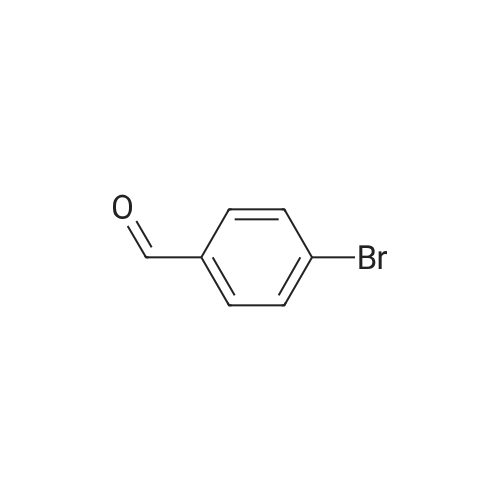
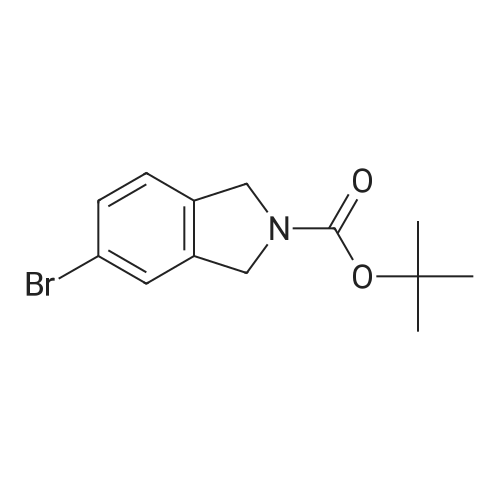
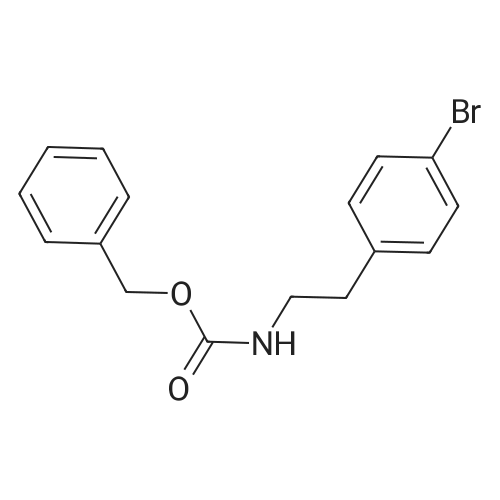
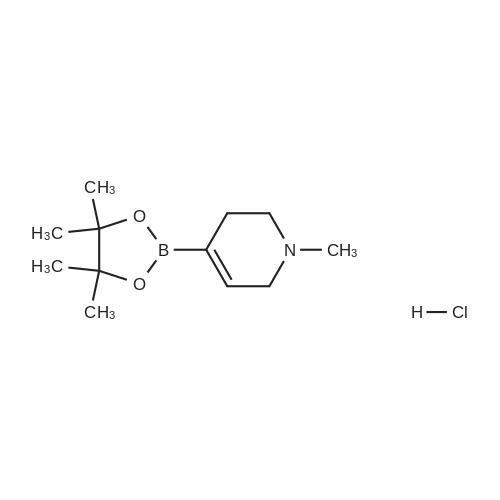
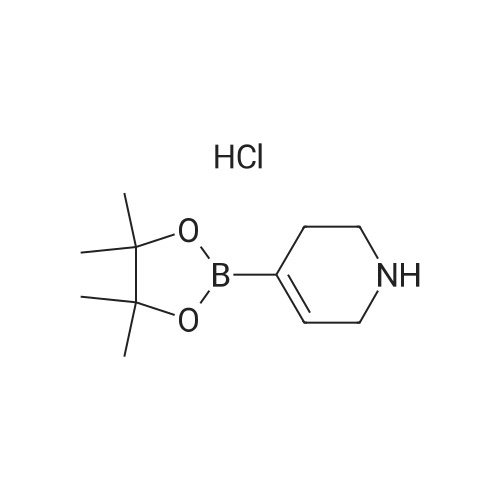
| 79% |
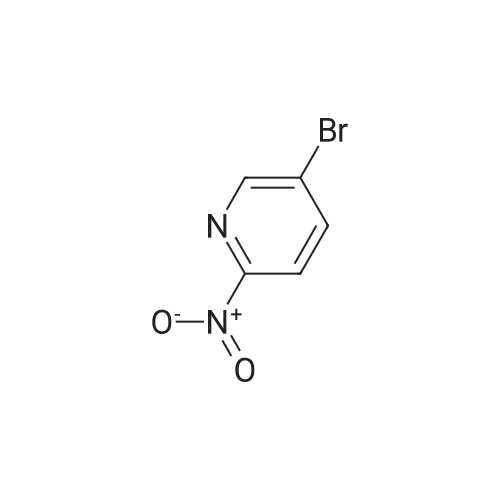
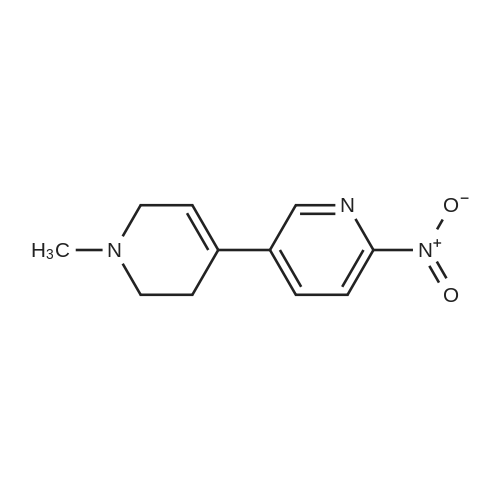
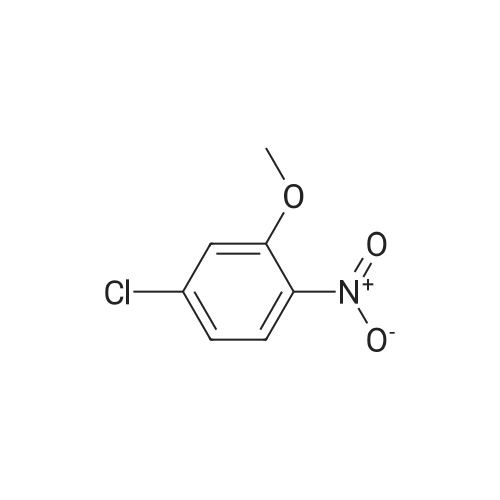
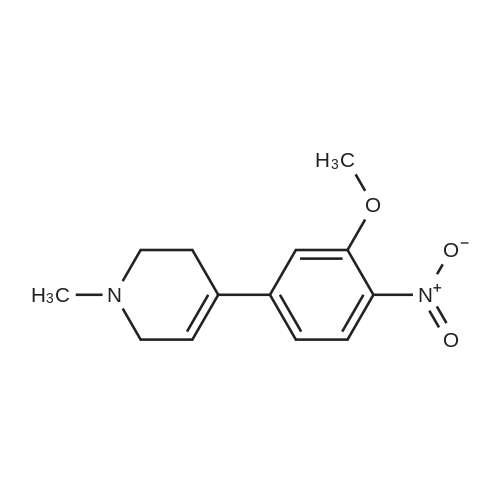
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; at 100℃; for 4h;Inert atmosphere; Sealed tube; |
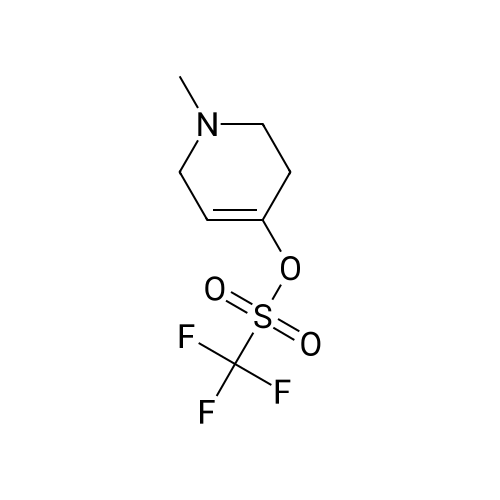
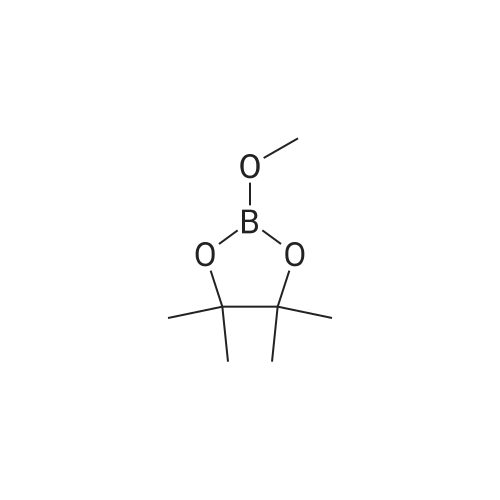
In a microwave pressure vessel equipped with a magnetic stirring bar was added (5 -7-chloro-3-(l,4-dimethyl-lH-l,2,3-triazol-5-yl)-5-((2-fluorophenyl)(tetrahydro-2H- pyran-4-yl)methyl)-5H-pyrrolo[2,3-b:4,5-b']dipyridine (30 mg, 0.061 mmol), l-methyl-4- (4,4,5, 5-tetramethyl-l,3,2-dioxaborolan-2-yl)-l,2,3,6-tetrahydropyridine (20.45 mg, 0.092 mmol), dioxane (2 mL) and water (1 mL). Potassium carbonate (21.1 mg, 0.153 mmol) and Pd(Ph3P)4 (5.3 mg, 4.58 muetaiotaomicron) was added. Argon was bubbled into the mixture for 5 min, then the vessel was capped and placed into a preheated oil bath at 100°C. The reaction mixture was stirred for 4 h. Solids in the reaction mixture were filtered, and the filtrate was purified by preparative HPLC: Column: Waters XBridge C18, 19 x 200 mm, 5-mupiiota particles; Mobile Phase A: 5:95 methanol: water with lOmM NH40Ac; Mobile Phase B: 95:5 methanol: water with lOmM NH4OAC; Gradient: 10- 50percent B over 30 min, then a 5-min hold at 100percent B; Flow: 20 mL/min. Fractions containing the desired product were combined and dried via centrifugal evaporation to give 27.3 mg (79percent) of the title compound with an average purity by LC/MS analysis was 97percent. Two analytical LC/MS injections were used to determine the final purity. Injection 1 conditions: Column: Waters BEH C18, 2.0 x 50 mm, 1.7mupiiota particles; Mobile Phase A: 5:95 ACN:water with 10 mM NH4OAc; Mobile Phase B: 95:5 ACN:water with 10 mM NILtOAc; Temperature: 50°C; Gradient: 0percentB, 0-100percent B over 3 min, then a 0.5-min hold at 100percent B; Flow: 1 mL/min; Detection: UV at 220 nm. Rt= 1.34 min; LC/MS (M+H) = 552.5. Injection 2 conditions: Column: Waters BEH CI 8, 2.0 x 50 mm, 1.7-mupiiota particles; Mobile Phase A: 5:95 methanol: water with 10 mM LiOAc; Mobile Phase B: 95:5 methanol: water with 10 mM NH4OAc; Temperature: 50°C; Gradient: 0percentB, 0-100percent B over 3 min, then a 0.5-min hold at 100percent B; Flow: 0.5 mL/min; Detection: UV at 220 nm. Rt= 2.77 min; LC/MS (M+H) = 552.5. NMR (500MHz, DMSO-c e) delta 8.60 (s, 1H), 8.54 (d, J=8.1 Hz, 1H), 8.47 (br. s., 1H), 8.22 (t, J=7.5 Hz, 1H), 7.64 (d, J=8.4 Hz, 1H), 7.32 (d, J=6.6 Hz, 1H), 7.28 - 7.21 (m, 1H), 7.12 (t, J=9.4 Hz, 1H), 6.96 (br. s., 1H), 6.17 (br. s., 1H), 4.04 (s, 3H), 3.94 - 3.85 (m, 1H), 3.74 (d, J=9.9 Hz, 1H), 3.65 (br. s., 1H), 3.47 - 3.41 (m, 1H), 3.26 - 3.19 (m, 1H), 3.17 (br. s., 2H), 2.80 (d, J=18.3 Hz, 2H), 2.69 (d, J=5.5 Hz, 2H), 2.32 (s, 3H), 2.35 (s, 3H), 1.87 (s, 1H), 1.63 (d, J=1 1.7 Hz, 1H), 1.53 - 1.41 (m, 1H), 1.35 (d, J=7.7 Hz, 1H), 1.00 (d, J=13.2 Hz, 1H). LC/MS (M+H) = 552.6; HPLC conditions: Rt = 2.45 min (Phenomenex LUNA CI 8 2 x 50 mm (4 min grad) eluting with 5-95percent aq ACN containing l OmM NH4OAC, 0.8 mL/min, monitoring at 254 nm); Temperature: 40°C). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping