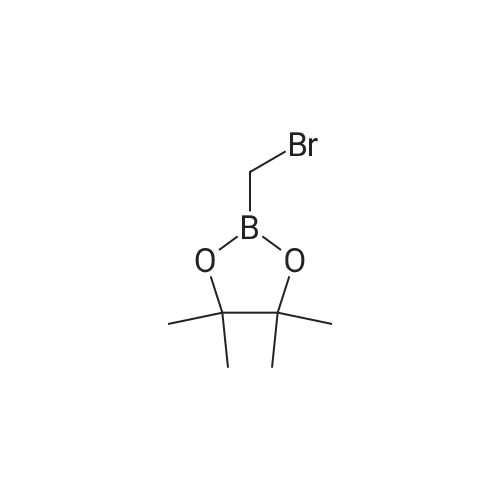
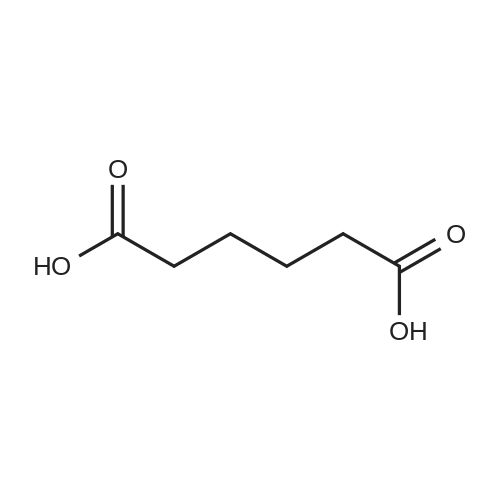
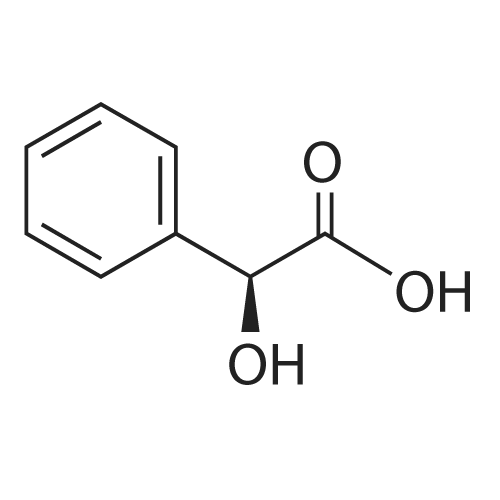
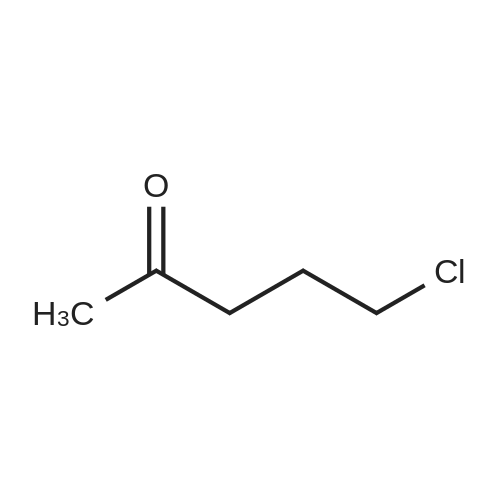
| 94.6% |
|
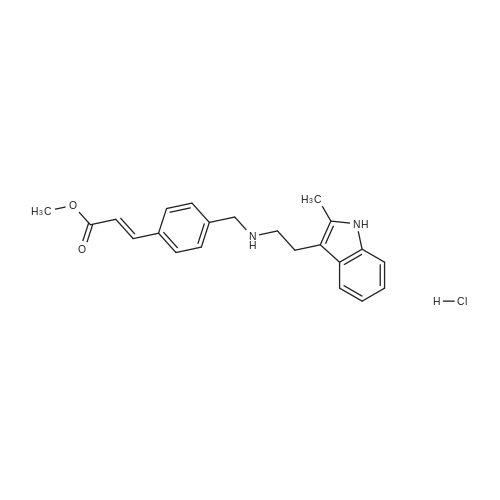
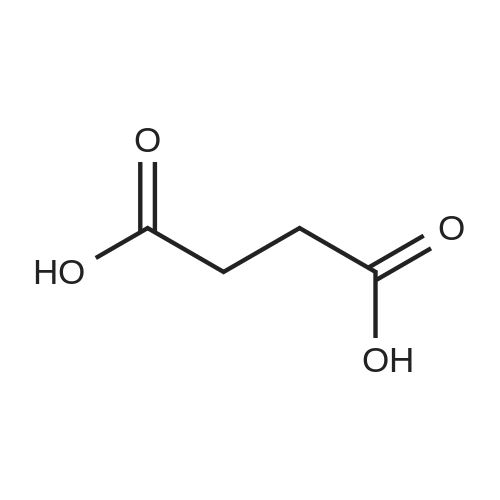
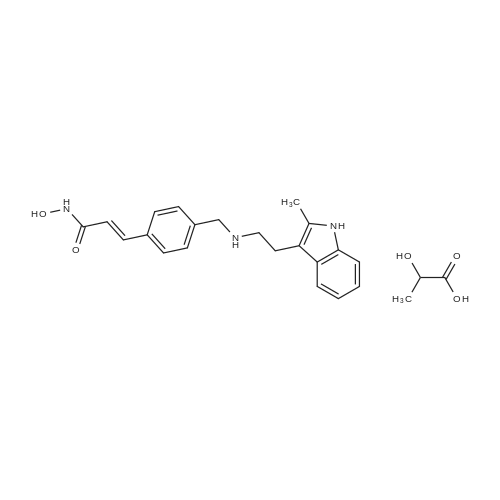
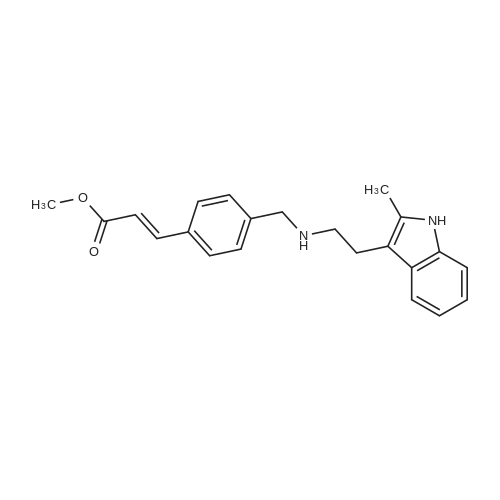
To a 250 ml four-necked flask was added (E) -3- [4 - [[2- (2-methyl-1H-indol-3-yl)(15 g, 0.039 mol), methanol (75 ml), cooled at -10 C to 15 C with stirring, and rapidly precipitated with sodium hydroxide (4.68 g, 0.71 m 1 ) In methanol solution, drop the mixture, stir lOmin, dropping hydroxylamine solution (32. 52g 50% aqueous solution corresponding to hydroxylamine hydrochloride 16.268,0.234111001), dropping finished at -10 (: - 15 (temperature insulation Stir for 2 hours.The reaction mixture was warmed to 0 C, kept at 0-5 C for 30 min, then warmed to 20 C, kept at 20-25 C for 1 hour, drop in water 38 ml, stir l0 min, Filtered and rinsed with 38 ml of water. The resulting filtrate was adjusted to pH 10 with an aqueous solution of hydrochloric acid (about 18. 5 g of an aqueous solution of 2 mol / L) to 10. The crystals were stirred at 20-25 C and treated with hydrochloric acid (About 15.5 g of 2 mol / L of aqueous solution), continue to adjust the PH value of the solution to 8-9, stirring at 20-25 C for 1 hour, continue to adjust the pH of the solution to 3-4, stirring for 1 hour , The solid was filtered and the filter cake was rinsed with 50 ml of a methanolic water mixture (nu / nu = 1: 1) and dried to a constant weight in a 50 hot air oven to give a solid 12. 89 g, yield 94. 6% : |
| 73% |
With hydroxylamine; potassium hydroxide; In methanol; at 0℃; for 4h;Inert atmosphere; |
A solution of potassium hydroxide (1.17 g, 21 mmol) in methanol (5 mL) was added to a stirred solution of hydroxylamine hydrochloride (0.97 g, 14 mmol) in methanol (10 mL) at 0 C. The mixture was stirred at 0 C for 15 min. The precipitate was removed by filtration and the filtrate was collected to provide fresh hydroxylamine solution. The ester (0.48 g, 1.4 mmol) was added to the above freshly prepared hydroxylamine solution at 0 C. The reaction mixture was then stirred at this temperature under a nitrogen atmosphere for 4 h. After the reaction was completed, the mixture was diluted with water and neutralised with NH4Cl aqueous solution to pH = 7-8. The precipitate that formed was collected by filtration, washed with water and recrystallised from MeOH/H2O to give the title compound as: Off-white solid; yield 0.35 g (73%); m.p. 89-91 C (lit. 1286-88 C); 1H NMR (600 MHz, DMSO-d6): delta 2.31 (s, 3H), 2.69 (t, J= 7.5 Hz, 2H), 2.81 (t, J= 7.5 Hz, 2H), 3.77 (s, 2H), 6.45 (d, J= 15.8 Hz, 1H), 6.90 (m, 1H), 6.95 (m, 1H), 7.22 (d, J= 8.0 Hz, 1H), 7.38 (m, 3H), 7.44 (d, J= 15.8 Hz, 1H), 7.49 (d, J= 8.0 Hz, 2H), 10.70 (brs, 1H); 13C NMR (150 MHz, DMSO-d6): delta 11.7, 24.7, 50.0, 52.8, 108.5, 110.8, 117.8, 118.4, 118.8, 120.3, 127.7, 128.8, 128.9, 132.2, 133.6, 135.6, 138.6, 142.7, 163.2; MS (ESI) m/z [M + H]+: 350.0; HRMS m/z calcd for C21H23O2N3[M + H]+: 350.1863; found: 350.1864. |
|
With sodium hydroxide; hydroxylamine hydrochloride; In water; at 0℃; for 6h; |
A suspension of LiAIH4 (17 g, 445 mmol) in dry THF(IOOO mL) is cooled to 0 0C and 2- methylindole-3-gryoxylamide (30 g, 148 mmol) is added in portions over 30 min. The mixture is stirred at room temperature for 30 min. and then maintained at reflux for 3 h. The reaction is cooled to 0 0C and treated with H2O (17ml), 15% NaOH (aq., 17ml) and H2O (51 ml). The mixture is treated with MgSO4, filtered and the filtrate evaporated to give 2-methyltryptamine which is dissolved in MeOH. Methyl 4-formylcinnamate (16.9 g, 88.8 mmol) is added to the solution, followed by NaBH3CN (8.4 g) and AcOH (1 equiv.). After 1 h the reaction is diluted with NaHCO3 (aq.) and extracted with EtOAc. The organic extracts are dried (MgSO4), filtered and evaporated. The residue is purified by chromatography to give 3-(4-[2-(2-methyl-1 /-/-indo.-3-yl)-ethylamino]-methyl}-phenyl)-(2£)-2-propenoic acid methyl ester. The ester is dissolved in MeOH, 1.0 M HCI/dioxane (1 - 1.5 EPO <DP n="27"/>eqiv.) is added followed by Et2O. The resulting precipitate is filtered and the solid washed with Et2O and dried thoroughly to give 3-(4-[2-(2-methyl-1 /-/-indol-3-yl)-ethylamino]-methyl}-phenyl)-(2£)-2- propenoic acid methyl ester hydrochloride. 1.0 M NaOH (aq., 85 mL) is added to an ice cold solution of the methyl ester hydrochloride (14.9 g, 38.6 mmoi) and HONH2 (50% aq. solution, 24.0 mL, ca. 391.2 mmol). After 6 h, the ice cold solution is diluted with H2O and NH4CI (aq., 0.86 M, 100 mL). The resulting precipitate is filtered, washed with H2O and dried to afford Lambda/-hydroxy-3-[4-[[[2-(2- methyl-1 W-indol-3-yl)-ethyl]-amino]methyl]phenyl]-2E-2-propenamide (m/z 350 [MH+]). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +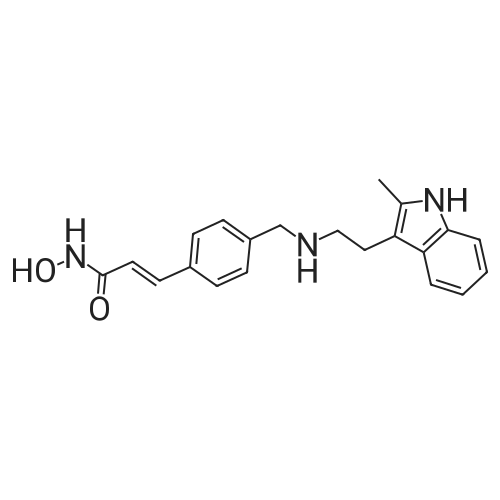

 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping