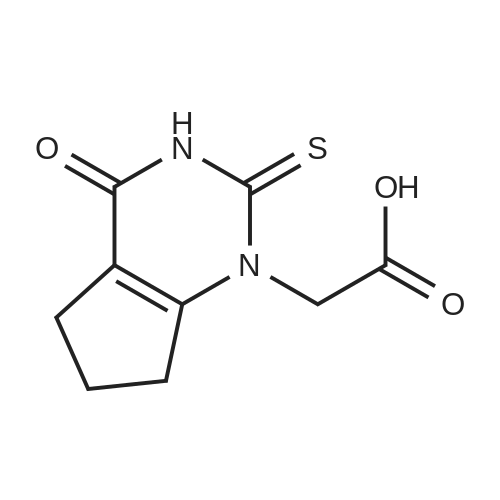
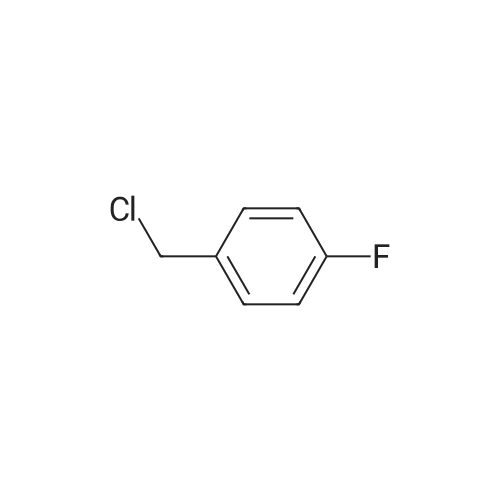
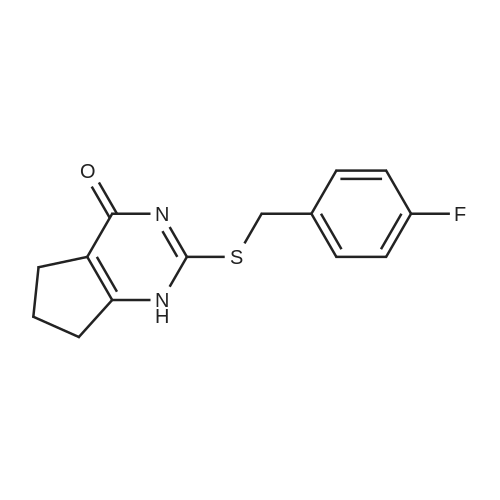
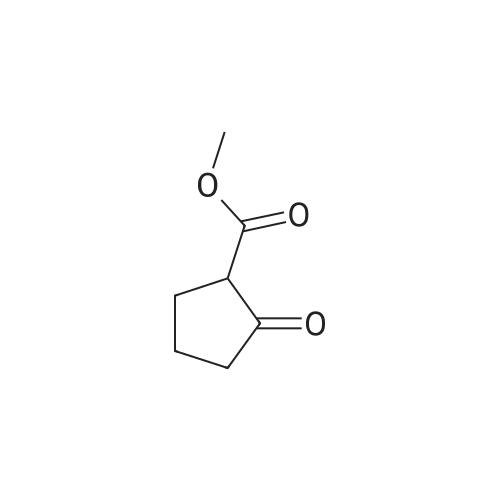
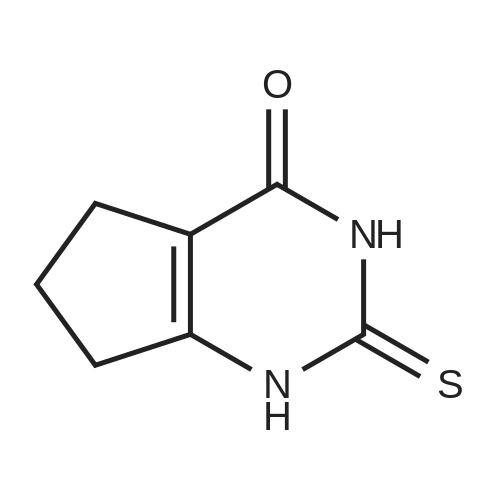
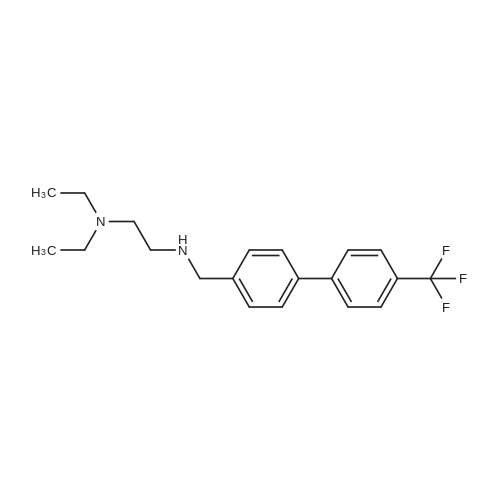
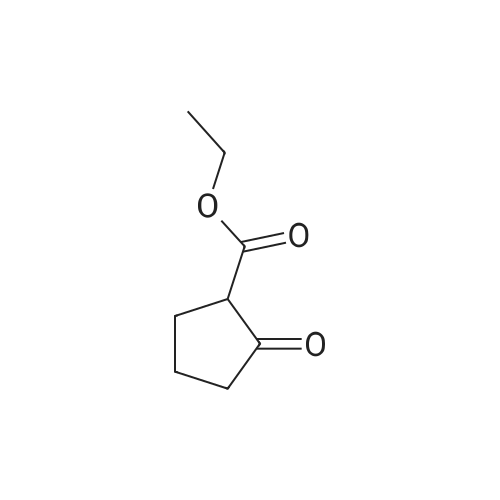
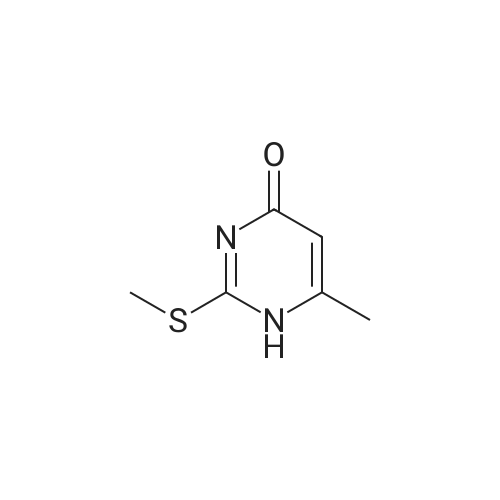
| 65% |
With 1-[(1-(cyano-?2-?ethoxy-?2-?oxoethylidenaminooxy)?dimethylamino-?morpholino)]-uronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 0 - 20℃; for 6h;Inert atmosphere; |
Amine 8 (1 eq, 52 mg) and acid 12' (1 eq, 50 mg) were dissolved in DMF (1 mL) then stirred with DIPEA (2 eq, 52 mu) for 10 min at rt. The reaction was then cooled with an ice bath to 0C and COMU (1 eq, 64 mg) was added. The mixture was stirred at 0C for lh then the bath was removed and the reaction was allowed to reach rt and continued for 5h. The compound was extracted using EtOAc three times (5 mL x 3). The combined organic layers were then washed several times with saturated NaHC03 until washing solution was colourless. The resulting organic solution was dried, filtered and evaporated. The product was then purified using column chromatography on silica gel DCM/MeOH (90/10) (Rf = 0.2) as a pale yellow solid (65 mg, 65%). 1H NMR (600 MHz, CDC13) Mix of rotamers ratio (1/1) delta 7.69 (d, J = 7.9 Hz, 2H), 7.62 (d, J = 7.9 Hz, 1H), 7.56 (d, J = 6.7 Hz, 1H), 7.46 (m, 2H), 7.37-7.26 (m, 4H), 6.96 (t, J = 7.4 Hz, 1H), 6.89 (t, J = 7.4 Hz, 1H), 4.95 (s, 1H), 4.69 (m, 3H), 4.50 (s, 1H), 4.40 (s, 1H), 3.64 (m, 1H), 3.30 (m, 1H), 2.88 (m, 1H), 2.81 (t, J = 6.7 Hz, 1H), 2.76 (t, J = 6.7 Hz, 4H), 2.59 (t, J = 7.1Hz, 2H), 2.51 (q, J = 7.0 Hz, 2H), 2.11 (quint, J = 7.4 Hz, 1H), 2.05 (quint, J = 7.4 Hz, 1H), 1.10 (t, J = 7.1Hz, 3H), 0.98 (t, J = 7.1Hz, 3H). 13C-NMR (CDC13, 150 MHz) Mix of rotamers delta: 167.5, 166.5, 162.2 (d, JCF = 240 Hz), 161.4, 161.3, 156.8, 156.5, 144.1, 143.6, 139.8, 139.3, 136.8, 135.4, 131.5 (d, JCF = 3 Hz), 131.2 (d, JCF = 9 Hz), 131.1 (d, JCF = 3 Hz), 129.8 (m), 128.8, 128.0 (m), 127.7 (m), 127.4, 127.3, 127.2, 125.9 (m), 124.4 (m), 121.3, 121.2, 115.7 (d, JCF = 23 Hz), 115.6 (d, JCF = 23 Hz), 51.4, 50.4, 50.3, 50.1, 49.2, 47.8, 47.3, 46.1, 36.6, 36.5, 32.1, 32.0, 28.5, 28.4, 20.9, 20.8, 11.8; |
|
|
Example 3(a) 1-(N-(2-(Diethylamino)ethyl)-N-(4-(4-trifluoromethylphenyl)benzyl)aminocarbonylmethyl)-2-(4-fluorobenzyl)thio-5,6-trimethylenepyrimidin-4-one Intermediate B69 (87.1 g, 0.26 mol.) was suspended in dichloromethane (2.9 liter). 1-Hydroxybenzotriazole hydrate (35.2 g, 0.26 mol.) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (99.7 g, 0.52 mol.) were added and the suspension stirred for 45 minutes by which time complete solution had been obtained. Intermediate A30 (91.2 g, 0.26 mol.) was added as a solution in dichloromethane (100 ml) over 5 minutes and the solution stirred for 4 hours. Saturated ammonium chloride solution:water mixture (1:1, 1 liter) was added and the solution stirred for 10 minutes. The organic phase was separated and extracted with saturated ammonium chloride:water mixture (1:1, 1 liter), extracts were pH 6. The organic phase was separated and extracted with water (1 liter) containing acetic acid (10 ml), extract pH 5. The dichloromethane layer was separated and extracted with saturated sodium carbonate solution:water:saturated brine mixture (1:3:0.2, 1 liter), pH 10.5, then with saturated brine:water mixture (1:1, 1 liter). The brown solution was dried over anhydrous sodium sulfate in the presence of decolourising charcoal (35 g), filtered and the solvent removed in vacuo to give a dark brown foam. The foam was dissolved in iso-propyl acetate (100 ml) and the solvent removed in vacuo. The dark brown gummy residue was dissolved in boiling iso-propyl acetate (500 ml), cooled to room temperature, seeded and stirred overnight. The pale cream solid produced was filtered off and washed with iso-propyl acetate (100 ml). The solid was sucked dry in the sinter for 1 hour then recrystallized from iso-propyl acetate (400 ml). After stirring overnight the solid fowled was filtered off, washed with iso-propyl acetate (80 ml) and dried in vacuo to give the title compound, 110 g, 63.5% yield. 1H NMR (CDCl3, ca 1.9:1 rotamer mixture) delta 0.99 (6H, t), 2.10 (2H, m), 2.50 (4H, q), 2.58/2.62 (2H, 2*t), 2.70/2.82 (2H, 2*t), 2.86 (2H, t), 3.28/3.58 (2H, 2*t), 4.45/4.52 (2H, 2*s), 4.68/4.70 (2H, 2*s), 4.93 (2H, s), 6.95 (2H, m), 7.31 (2H, d), 7.31/7.37 (2H, 2*m), 7.48/7.52 (2H, d), 7.65 (2H, m), 7.72 (2H, m); MS (APCI) (M+H)+ 667; mp 125 C. (by DSC-assymetric endotherm). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping