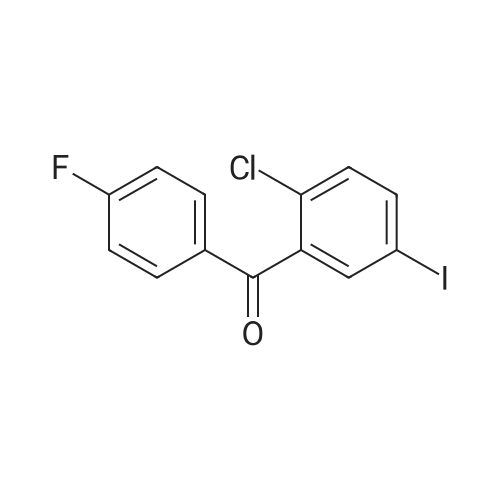
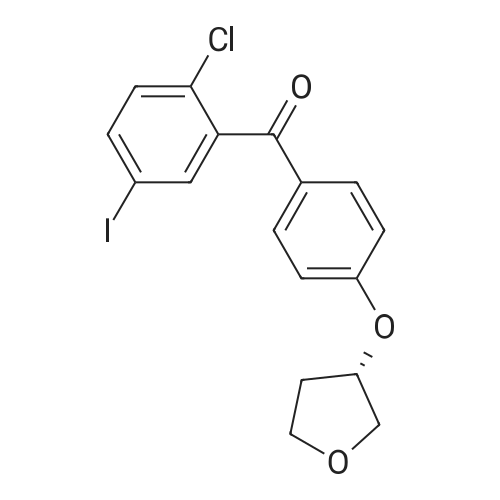
|
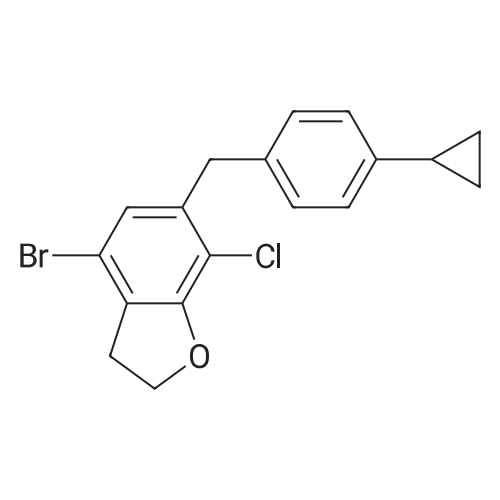
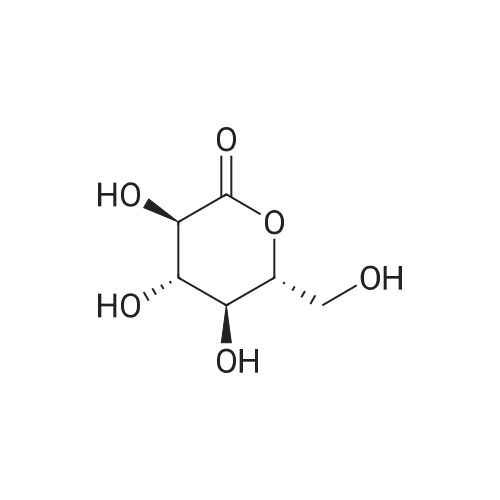
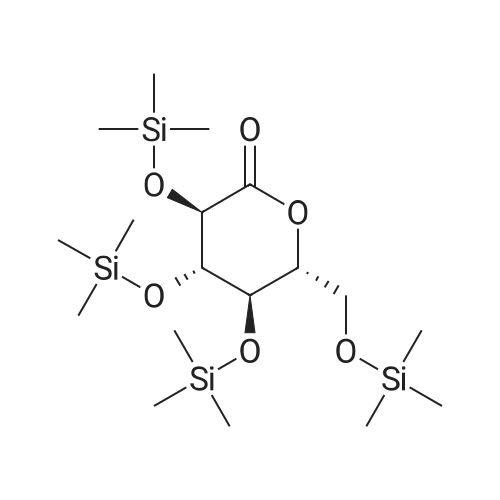
Stage #1: 4-bromo-7-chloro-6-(4-cyclopropylbenzyl)-2,3-dihydrobenzofuran; (3R,4S,5R,6R)-3,4,5-tris((trimethylsilyl)oxy)-6-(((trimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-2-one With n-butyllithium In tetrahydrofuran; hexane at -78 - 20℃; for 1.66667h; Inert atmosphere;
Stage #2: methanol With hydrogenchloride In tetrahydrofuran; hexane; water at -30 - 20℃; for 18h; |
6.1a; 6.1b; 7.1 (1b)
To a reaction vessel were added dropwise 4-bromo-7-chloro-6-(4-cyclopropylbenzyl)-2,3-dihydrobenzofuran (10.0 g, 27.5 mol), (3R,4S,5R,6R)-3,4,5-tris((trimethylsilyl)oxy)-6-(((trimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-2-one (25.7 g, 54.9 mol) and anhydrous tetrahydrofuran (80 mL) at room temperature under nitrogen, and the mixture was completely dissolved. After the reaction vessel was cooled to -78° C., n-butyllithium (22.1 mL, 2.5 M in hexane, 54.9 mmol) was added dropwise thereto for 15 minutes while keeping the internal temperature at -60° C. or lower. Once the dropwise addition of n-butyllithium was complete, the mixture was further stirred at -78° C. for 30 minutes. To the reaction mixture was added dropwise a concentrated hydrochloric acid/methanol (7.01 mL/70 mL) solution for 10 minutes while keeping the internal temperature at -30° C. or lower. Once the dropwise addition was complete, the reaction vessel was moved to room temperature and stirred for 18 hours. After confirming completion of the reaction, the reaction vessel was cooled to 0° C., a saturated NaHCO3 aqueous solution (60 mL) was added thereto, the pH was adjusted to 9 to 10 using a pH meter, and then the reaction solvent was removed using a vacuum concentrator. The concentrate was diluted with EtOAc (60 mL), distilled water (60 mL), and brine (60 mL), and layered. Then, the organic layers were pooled and the aqueous layer was extracted with EtOAc (2×30 mL). The organic layers were combined and rinsed with distilled water (60 mL) and brine (60 mL). The organic layer was dried over MgSO4 (5 g), filtered, and the filtrate was concentrated under reduced pressure to remove the solvent. The residue was diluted with toluene (50 mL), and then the toluene solution was slowly added dropwise to hexane (200 mL) at room temperature while stirring the hexane. The resulting suspension was stirred at the same temperature for 1 hour and then filtered in vacuo. The resulting filtrate was washed with hexane (10 mL), and then dried in a vacuum oven (40° C.) until a moisture content thereof became 1% or lower through a Karl-Fischer analysis, to obtain the title compound (12.6 g, 96%) as a yellow solid. 1H NMR (500 MHz, CDCl3): δ 7.02 (d, J=8.0 Hz, 2H), 6.92 (d, J=8.0 Hz, 2H), 6.81 (s, 1H), 4.64 (m, 1H), 4.57 (m, 1H), 4.05 (d, J=15.0 Hz, 1H), 3.96 (d, J=15.0 Hz, 1H), 3.93 (dd, J=11.8, 3.0 Hz, 1H), 3.87 (m, 2H), 3.65 (m, 2H), 3.51 (m, 1H), 3.30 (d, J=9.5 Hz, 1H), 3.14 (s, 3H), 1.83 (m, 1H), 0.91 (m, 2H), 0.63 (m, 2H); LC-MS: [M-OMe]+ 445. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping