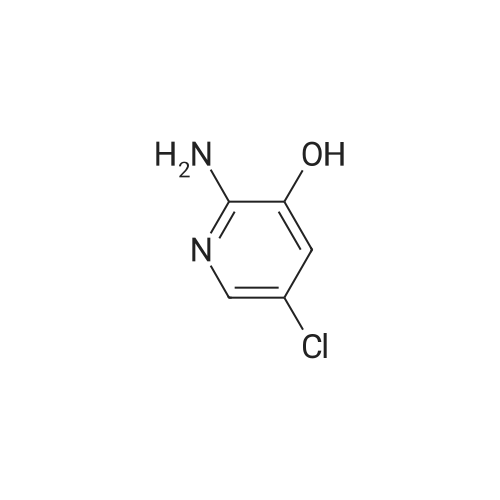
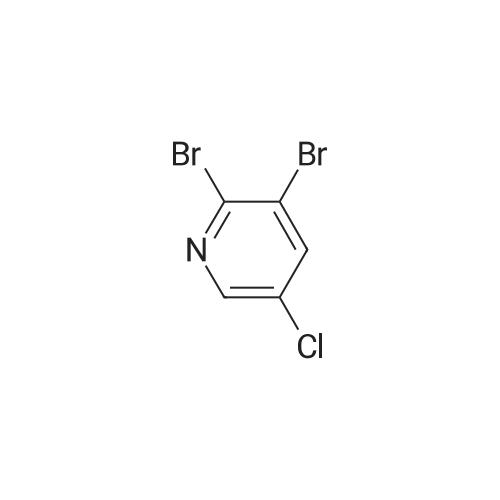
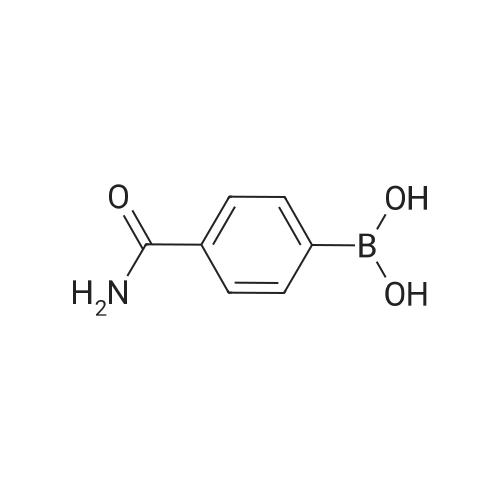
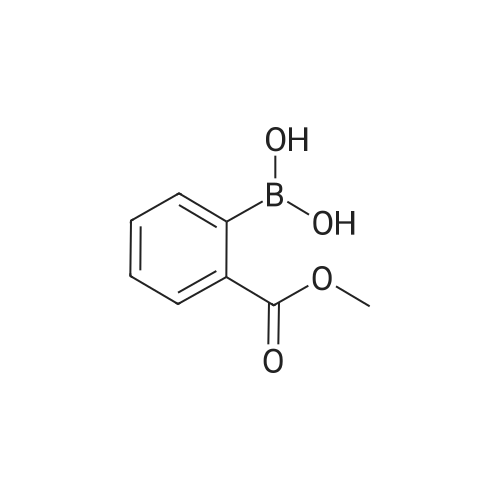
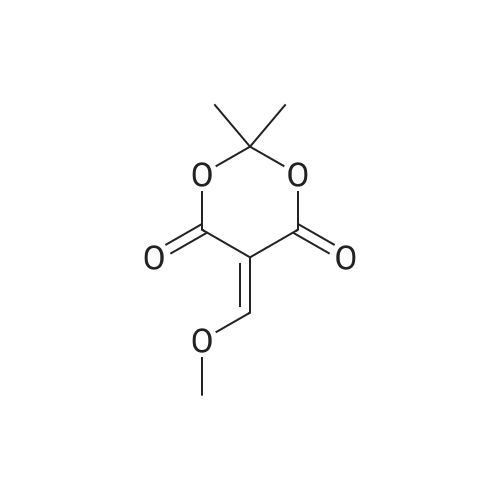
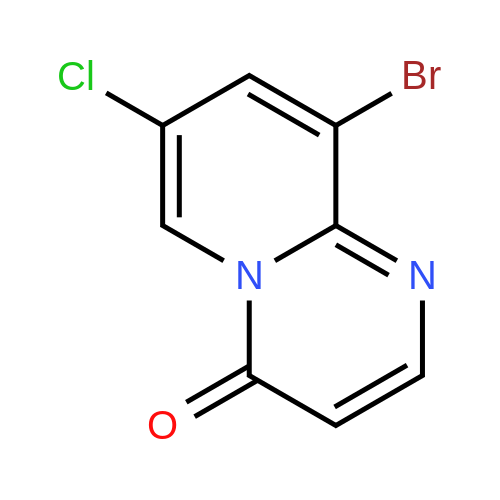
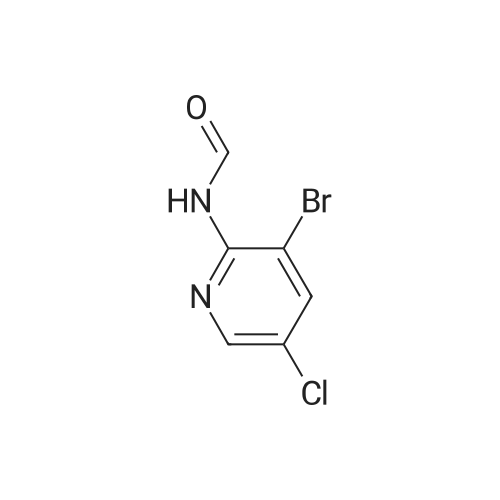
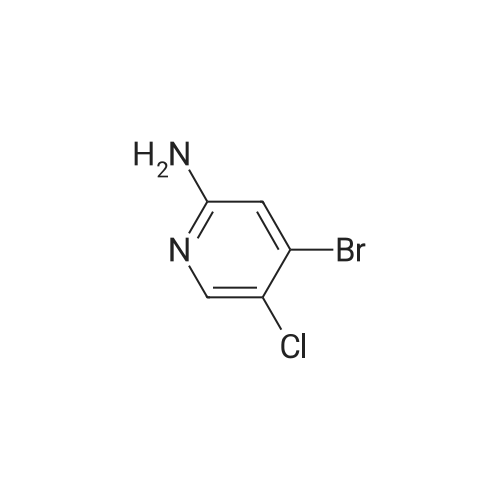
| 71% |
With N-Bromosuccinimide; In acetonitrile; at 80℃; for 1h; |
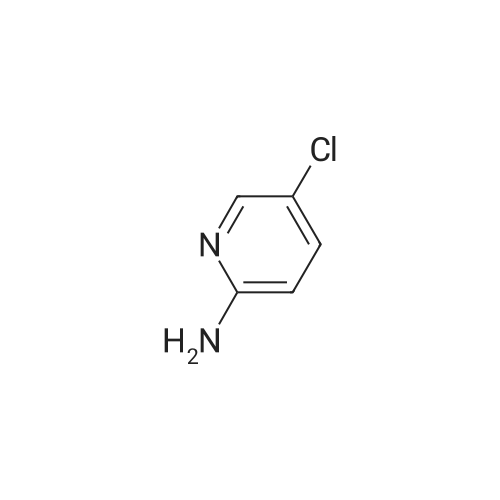
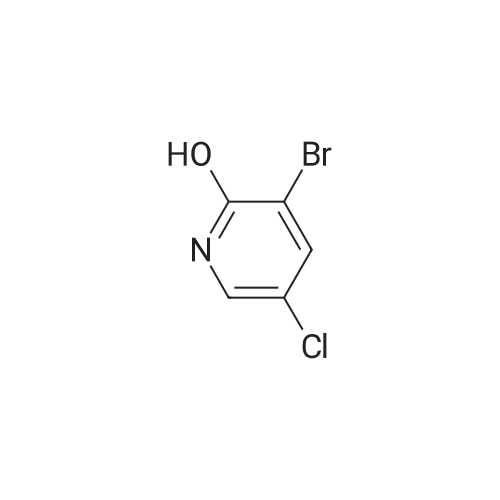
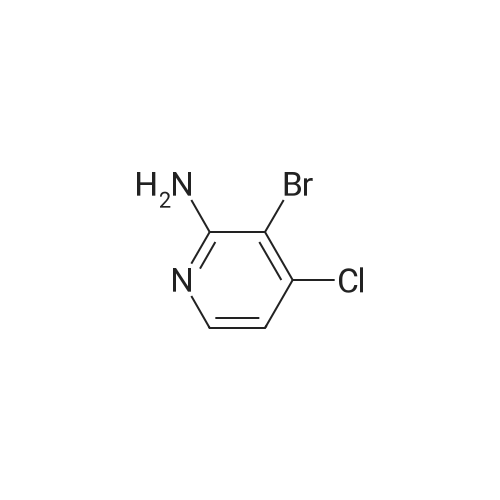
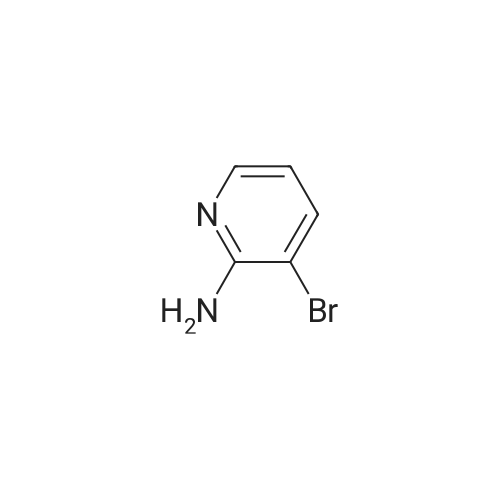
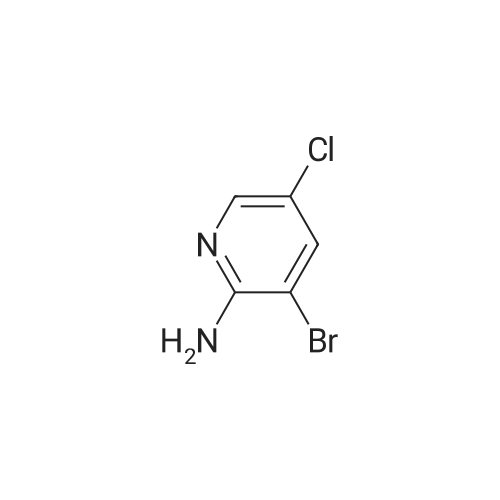
To a solution of 9 5-chloropyridin-2-amine (10g, 77.8mmol, 1 equiv.) in 31 acetonitrile (150mL), 10 N-bromosuccinimide (13.8g, 77.8mmol, 1 equiv.) was added. The reaction mixture was stirred and refluxed for 1h. Then the solvent was evaporated in vacuo. Compound 11 1 was obtained, after purification by chromatography on silica gel (eluent: dichloromethane?ethyl acetate 9:1) and recrystallization from propan-2-ol as a pale beige solid in 71percent yield. mp: 82°C, Lit.: 83?84°C; 1H NMR (400MHz, [D6]DMSO) delta: 6.43 (2H, s), 7.88 (1H, d, J=2.2Hz), 7.96 (1H, d, J=2.2Hz), 13C NMR (100MHz, [D6]DMSO) delta: 103.0 (C), 117.1 (C), 139.0 (CH), 145.0 (CH), 155.3 (C); MS ESI+ (m/z): 207.27/209.32 [M+H]+. |
| 70.4% |
With N-Bromosuccinimide; In ethanol; N,N-dimethyl-formamide; at 85℃; for 3.5h; |
DMF and ethanol was added in a 50L round-bottomed three-necked flask volume ratio of 1: mixed solvent 18.7L 1.8, insert the thermometer and reflux condenser installation means, start with a magnetic stirrer, and adding 4986.6g of 2-amino-5- chloro pyridine, N- bromosuccinimide 17336.3g, at 85 reaction was stirred 3.5 hours, GC and TLC determined the completion of the reaction, the solvent was removed by rotary evaporation to give the crude product from n-hexane to give the pure product recrystallized from 2- amino-3-bromo-5-chloropyridine, after drying, the yield was calculated 70.4percent, a purity of 99.15percent. |
| 48% |
With sodium hydroxide; bromine; sodium acetate; In water; acetic acid; |
Example 2 A suspension of 2-amino-5-chloropyridine (5.14 g, 40 mmol) and anhydrous sodium acetate (3.29 g, 40 mmol) in acetic acid (25 mL) was mechanically stirred and warmed to a bath temperature of 45° C. A solution of bromine (2.1 mL, 40 mmol) in acetic acid (2 mL) was added over 1 hour. The resulting orange mixture was cooled to 15° C. and filtered. The solids were taken up in 400 mL of water, the suspension made basic by addition of 1 N NaOH and the suspension extracted with ethyl acetate (5*100 mL). The combined extracts were washed with 10percent NaHSO3(1*100 mL) and were dried over MgSO4. Concentration afforded 2-amino-3-bromo-5-chloropyridine (4 g, 48percent), mp 82-84° C. |
|
With sodium hydroxide; bromine; In water; acetic acid; ethyl acetate; |
1.1 2-Amino-3-bromo-5-chloropyridine A solution of 257 g (1.61 mol) of bromine in 380 ml of acetic acid was added dropwise over a period of one hour to a solution of 187 g (1.45 mol) of 2-amino-5-chloropyridine in 1.5 of acetic acid. The reaction mixture was refluxed for 3 h and then allowed to cool to ambient temperature. 1-9 l of demineralized water were added and the mixture was concentrated in vacuo. The residue was partitioned between 4 l of ethyl acetate and 1.8 l of water. The mixture was basified with 3 l of a 10percent by weight aqueous sodium hydroxide solution. The organic layer was separated and washed with brine (2*1.5 l), dried over magnesium sulfate, filtered and concentrated in vacuo. The residue was filtered, washed with hexane (2*100 ml) and dried in vacuo to give 192 g of 2-amino-3-bromo-5-chloropyridine. The mother liquor was concentrated in vacuo, filtered, washed with hexane and dried to give further 75 g of the same product. |
|
With sodium hydroxide; bromine; In acetic acid; ethyl acetate; |
Step 1: 2-Amino-3-bromo-5-chloropyridine To a solution of 2-amino-5-chloropyridine (10 g) in acetic acid (75 mL) at r.t. was added bromine (2.6 mL) slowly. After 30 min, the acid was neutralized by the careful addition of sodium hydroxide (10N) at 0° C. The resulting orange precipitate was dissolved in ethyl acetate and washed successively with saturated potassium carbonate, saturated Na2 S2 O3 and brine, dried and concentrated. Flash chromatography (eluding with hexane/ethyl acetate, 3:1 v/v) of the residual solid provided the title compound as a pale yellow solid (14.8 g). |
|
With sodium hydroxide; bromine; In acetic acid; ethyl acetate; |
Step 1 2-Amino-3-bromo-5-chloropyridine To a solution of 2-amino-5-chloropyridine (10 g) in acetic acid (75 mL) at r.t. was added bromine (2.6 mL) slowly. After 30 min, the acid was neutralized by the careful addition of sodium hydroxide (10 N) at 0° C. The resulting orange precipitate was dissolved in ethyl acetate and washed successively with saturated potassium carbonate, saturated Na2 S2 O3 and brine, dried and concentrated. Flash chromatography (eluding with hexane/ethyl acetate, 3:1 v/v) of the residual solid provided the title compound as a pale yellow solid (14.8 g). |
|
With sodium hydroxide; bromine; In acetic acid; ethyl acetate; |
Step 1 2-Amino-3-bromo-5-chloropyridine To a solution of 2-amino-5-chloropyridine (10 g) in acetic acid (75 mL) at r.t. was added bromine (2.6 mL) slowly. After 30 min, the acid was neutralized by the careful addition of sodium hydroxide (10 N) at 0° C. The resulting orange precipitate was dissolved in ethyl acetate and washed successively with saturated potassium carbonate, saturated Na2 S2 O3 and brine, dried and concentrated. Flash chromatography (eluding with hexane/ethyl acetate, 3:1 v/v) of the residual solid provided the title compound as a pale yellow solid (14.8 g). |
| 102.1 g (70.7%) |
With bromine; sodium acetate; In water; acetic acid; |
EXAMPLE 1 2-Amino-3-bromo-5-chloropyridine An acetic acid (400 mL) suspension of sodium acetate (57.1 g, 0.696 mol) and 2-amino-5-chloropyridine (89.5 g, 0.696 mol) was treated with a solution of bromine (35.9 mL, 0.696 mol) in acetic acid (15 mL) over a period of 1.25 hours with gentle warming to 43° C. The resulting orange slurry was then cooled to 15° C. and filtered to provide a solid which was subsequently dissolved in water, basified (pH 8.5) with 1N NaOH, and extracted with ethyl acetate (5*150 mL). The combined organic layers were washed with 10percent sodium bisulfite (2*150 mL), dried over magnesium sulfate, and condensed to give 102.1 g (70.7percent) of a yellow solid, m.p. 83-85° C.: IR(CHCl3) 3683, 3504, 3401, 2987, 2482, 2392, 1608, 1571, 1544, 1455, 1389, 1316, 1207, 1127, 1057, 1026, 895, 881, 650, 607, 249, 236 cm-1; 1 H NMR (300 MHz, CDCl3) delta 5.20 (br s, 2H), 7.70 (d, 1H), J=2.2 Hz), 8.0 (d, 1H, J=2.2 Hz); 13 C NMR (CDCl3) delta 104.08, 120.33, 139.62, 145.24, 154.10; exact mass calculated for C5 H4 N2 BrCl=205.9246. High resolution mass spec. 205.9246. |
|
With bromine; In dichloromethane; |
1.1. 2-Amino-3-bromo-5-chloropyridine 20 g (77.8 mmol) of 2-amino-5-chloropyridine dissolved in 160 ml of CH2 Cl2 are reacted with 8 ml of bromine, which is added dropwise at 0° C. After the addition is complete, the mixture is left to react at 25° C. for 2 h. The suspension is washed with 10percent strength sodium hydroxide solution, and the organic phase is washed with water and then dried over MgSO4, filtered and evaporated. The compound obtained is recrystallized in isopropanol. M.p. 82° C. |
|
With bromine; In acetic acid; at 20℃; for 0.5h; |
To a solution of 2-amino-5-chloropyridine (10 g) in acetic acid (75 mL) at r.t. was added bromine (2.6 mL) slowly. After 30 min, the acid was neutralized by the careful addition of sodium hydroxide (10 N) at 0°C. The resulting orange precipitate was dissolved in ethyl acetate and washed successively with saturated potassium carbonate, saturated Na2S2O3 and brine, dried and concentrated. Flash chromatography (eluting with hexane/ethyl acetate, 3:1 v/v) of the residual solid provided the title compound as a pale yellow solid (14.8 g). |
|
With bromine; acetic acid; at 18 - 25℃; for 0.5h; |
EXAMPLE 35-Chloro-3-(4-methylsulfonyl)phenyl-2-piperidin-1-ylpyridineStep 1: 2-Amino-3-bromo-5-chloropyridine To a solution of 2-amino-5-chloropyridine (10 g) in acetic acid (75 mL) at r.t. was added bromine (2.6 mL) slowly. After 30 min, the acid was neutralized by the careful addition of sodium hydroxide (10 N) at 0°C. The resulting orange precipitate was dissolved in ethyl acetate and washed successively with saturated potassium carbonate, saturated Na2S2O3 and brine, dried and concentrated. Flash chromatography (eluting with hexane/ethyl acetate, 3:1 v/v) of the residual solid provided the title compound as a pale yellow solid (14.8 g). |
| 2.75 g |
|
10716] To a solution of 5-chloropyridin-2-amine (5.0 g) in acetic acid (90 mE) was added dropwise a solution ofbromine (12.4 g) in acetic acid (4 mE) at 10° C. The reaction solution was stirred at room temperature for 2 hr, and saturated aqueous sodium thiosulfate solution was added thereto at 0° C. The mixture was stirred for 30 mm, the solvent was evaporated under reduced pressure, and the residue was extracted with ethyl acetate. The obtained organic layer was washed successively with saturated aqueous sodium bicarbonate solution and saturated brine, and dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate/hexane) to give the title compound (2.75 g).10717] ?H NMR (300 MHz, CDC13) oe 4.92 (2H, brs), 7.66 (1H, d, J=2.3 Hz), 7.98 (1H, d, J=2.3 Hz). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping