| 55% |
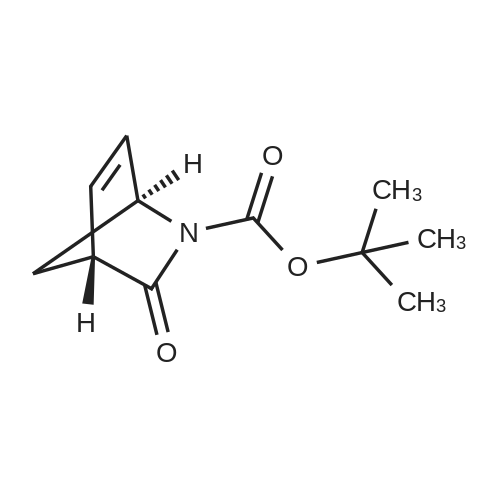
With N-ethyl-N,N-diisopropylamine; HATU; In N,N-dimethyl-formamide; at 0℃; for 1.5h; |
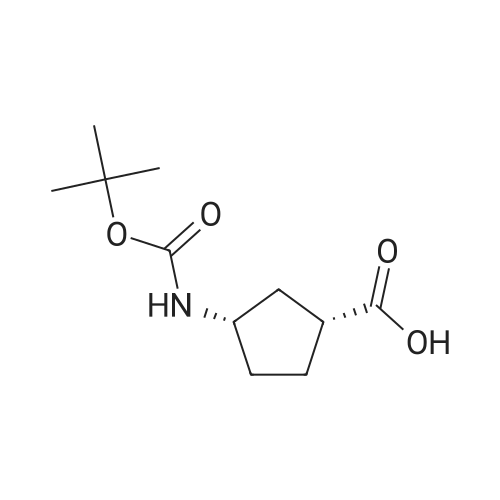
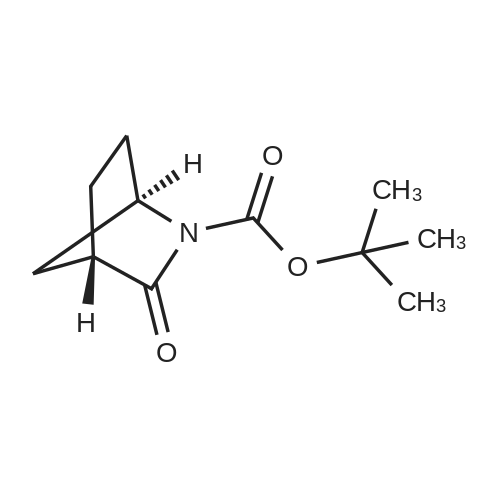
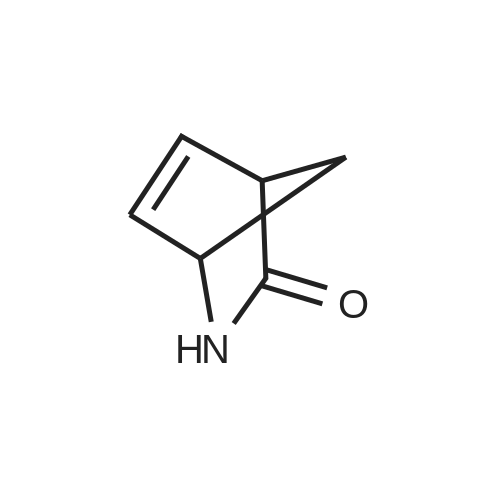
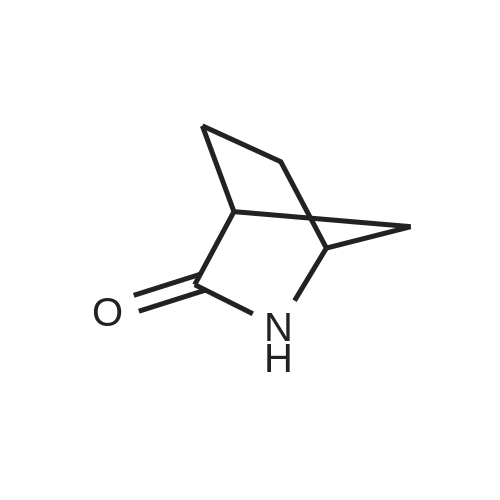
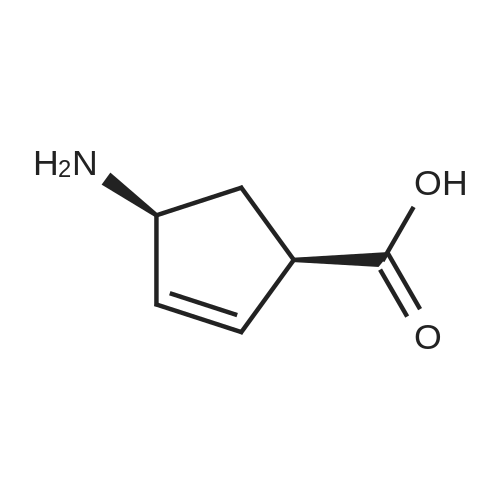
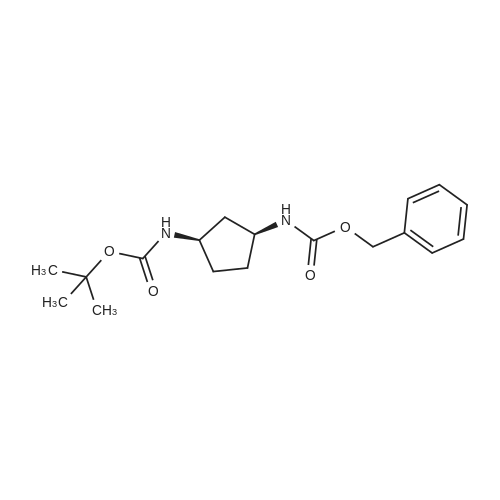
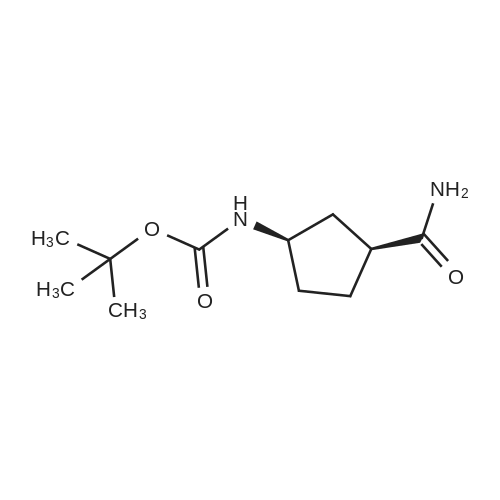
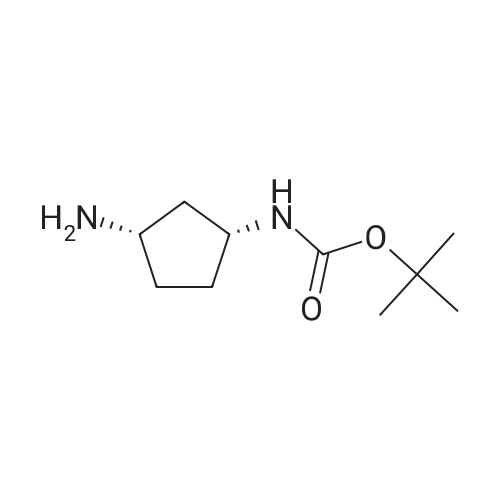
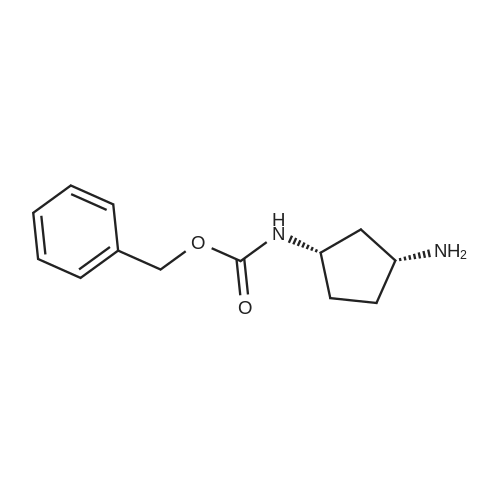
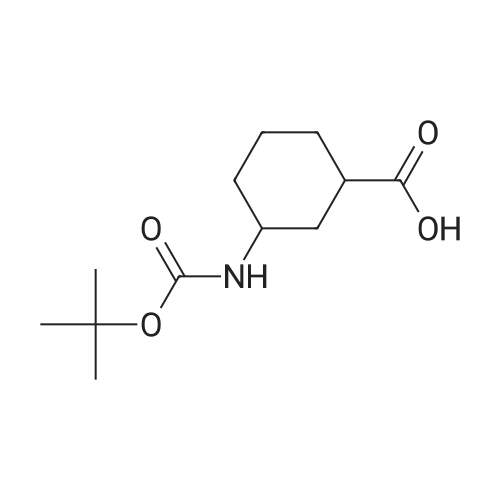
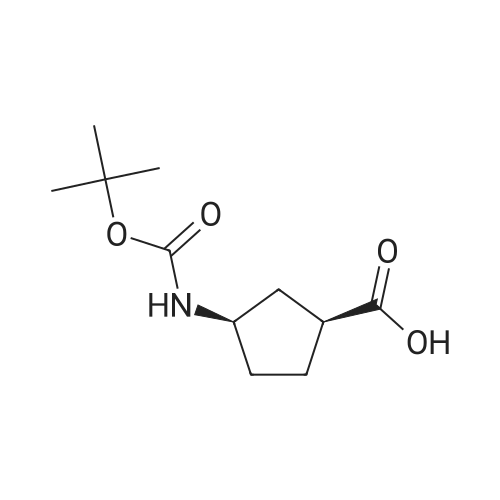
Example 26: Synthesis of '(2S,5R)-2-(5-(( 1 S,3R)-3 -aminocyclopentyl)- ,3 A-oxadiazol-2-yl)- -oxo-1 ,6-diazabicyclot 3.2.1 loctan-6-yl hydrogen sulfate ( Compound 729) Step 1: Synthesis of tert-butyl(lR,3S)-3-(2-((2S,5R)-6-(benzyloxy)-7 -oxo-1 ,6-diaza-bicyclo [3.2.1 ]octane-2-carbonyl)hydrazinecarbonyl)cyclopentylcarbamate: To a O C solution of (15',3 ?)-3-(ieri-butoxycarbonylamino)cyclopentanecarboxylic acid (3.8 g, 16.6 mmol) and (25,5 ?)-6-(benzyloxy)-7-oxo-l,6-diaza-bicyclo[3.2.1]octane-2- carbohydrazide (5.3 g, 18.26 mmol) in DMF (30 mL) were added HATU (7.6 g, 19.92 mmol) and DIPEA (11.6 ml, 66.4 mmol). The reaction mixture was stirred at 0 C for 1.5 hrs. The reaction mixture was diluted with water and extracted with EtOAc (2x). The combined organic layer was washed with saturated sodium chloride (4x) and citric acid (5% aq.), dried over Na2S04, and concentrated. The residue was purified by silica gel column (gradient elution 50-80% EtOAc/petroleum ether ) to afford ieri-butyl (lR,3S)-3-(2-((2S,5R)-6- (benzyloxy)-7-oxo- 1 ,6-diaza-bicyclo [3.2.1 ]octane-2-carbonyl) hydrazinecarbonyl) cyclopentylcarbamate (4.5 g, 55%) as a white solid. ESI-MS (EpsilonGamma, m/z): 502.3 [M+H]+. Step 2: Synthesis oftert-butyl(lR,3S)-3-(5-((2S,5R)-6-(benzyloxy)-7-oxo-l,6-diaza-bicyclo [3.2.1 ]octan-2-yl)-l,3,4-oxadiazol-2-yl)cyclopentylcarbamate: To a 0 C solution of PPh3 (2.2 g 8.4 mmol) in dry DCM (20 mL) was added I2 (2.1 g, 8.4 mmol). After I2 was dissolved, TEA (2.4 mL, 16.8 mmol) was added quickly at rt. The reaction mixture was stirred for 15 min. ieri-Butyl (l/?,35')-3-(2-((25',5 ?)-6-(benzyloxy)-7- oxo- l,6-diaza-bicyclo[3.2.1]octane-2-carbonyl)hydrazinecarbonyl) cyclopentylcarbamate (2.1 g, 4.2 mmol) was added. The reaction mixture was stirred at rt for 1 h. The reaction mixture was concentrated and the residue was purified by silica gel column (gradient elution 0-50% EtOAc/petroleum ether ) to afford the crude teri-butyl (lR,3S)-3-(5-((2S,5R)-6- (benzyloxy)-7-oxo- 1 ,6-diaza-bicyclo [3.2.1 ]octan-2-yl)- 1 ,3 ,4-oxadiazol-2-yl) cyclopentylcarbamate (-1.7 g) as a white solid. ESI-MS (EI+, m/z): 484.1. Step 3-5: Following Steps 3-5 in Example 4, replacing ieri-butyl (2-(5-((2S,5R)-6- (benzyloxy)-7-oxo-l,6-diazabicyclo[3.2.1]octan-2-yl)-l ,3,4-oxadiazol-2-yl)ethyl)carbamate in Step 3 with ieri-butyl ((lR,3S)-3-(5-((2S,5R)-6-(benzyloxy)-7-oxo-l,6- diazabicyclo [3.2.1 ]octan-2-yl)- 1 ,3 ,4-oxadiazol-2-yl)cyclopentyl)carbamate; (2S,5R)-2-(5 - ((lS,3R)-3-aminocyclopentyl)-l,3,4-oxadiazol-2-yl)-7-oxo-l ,6-diazabicyclo[3.2.1]octan-6-yl hydrogen sulfate (286 mg) was obtained as a white solid after prep-HPLC purification using ammonium formate buffer. ESI-MS (EI+, m/z): 314.2. lU NMR (300 MHz, D20) delta 4.74 (d, J = 6.5 Hz, 1H), 4.16 (br s, 1H), 3.82 - 3.66 (m, 1H), 3.56 - 3.41 (m, 1H), 3.15 (br d, / = 12.3 Hz, 1H), 2.89 (d, / = 12.3 Hz, 1H), 2.67 - 2.51 (m, 1H), 2.30 - 1.69 (m, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping