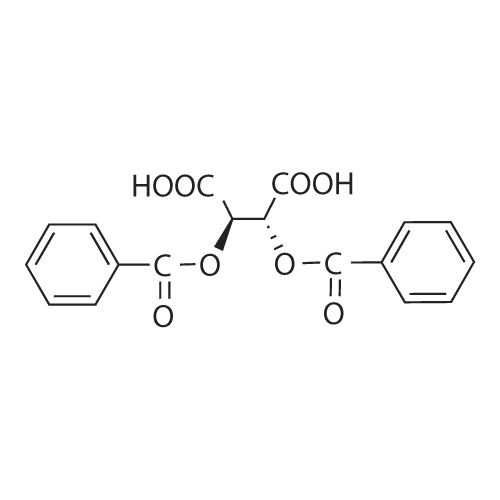
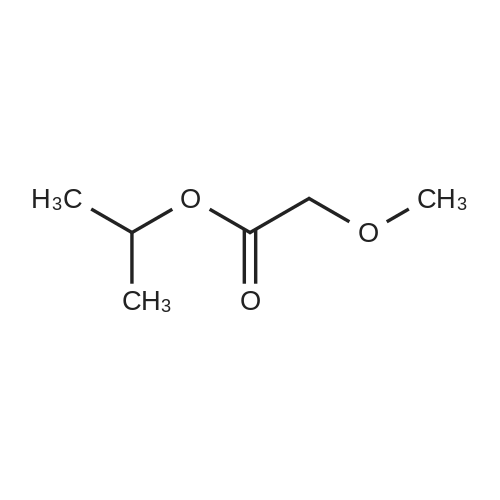
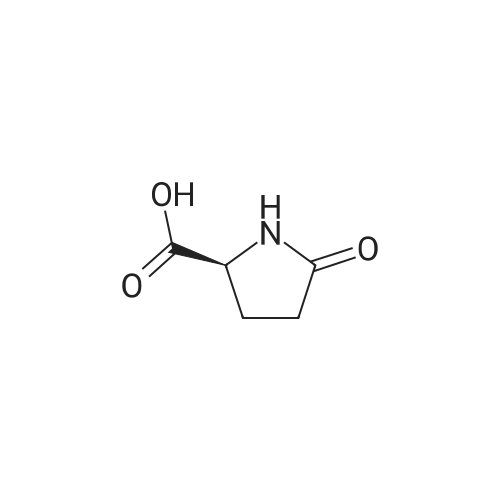
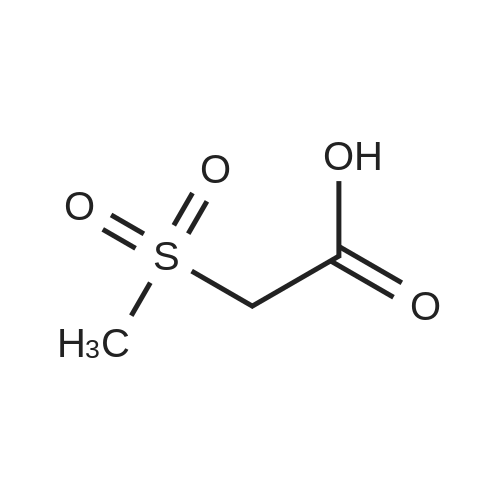
| 59% |
With acetic acid; for 15h;Reflux; |
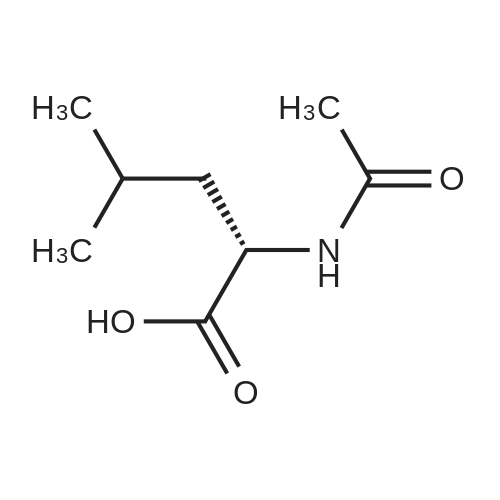
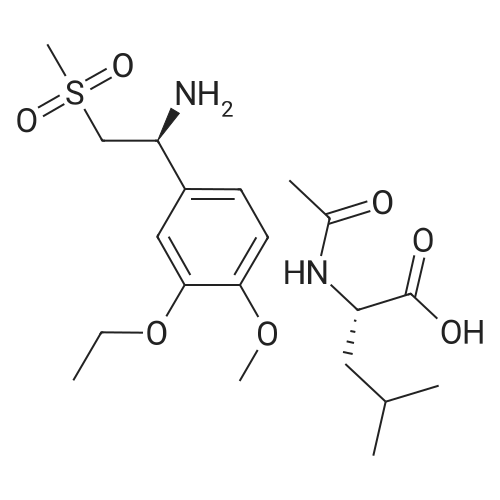
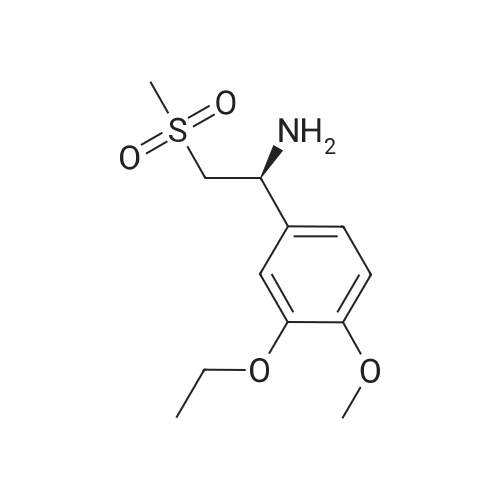
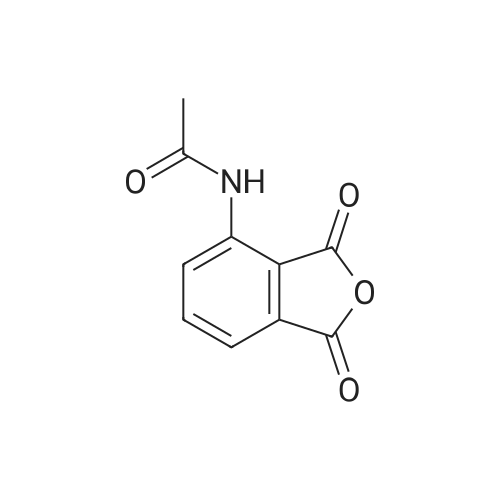
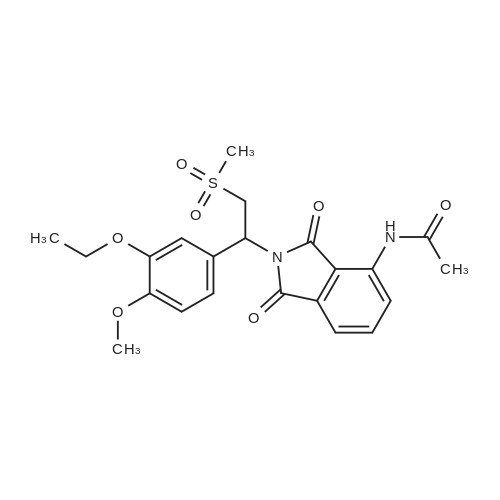
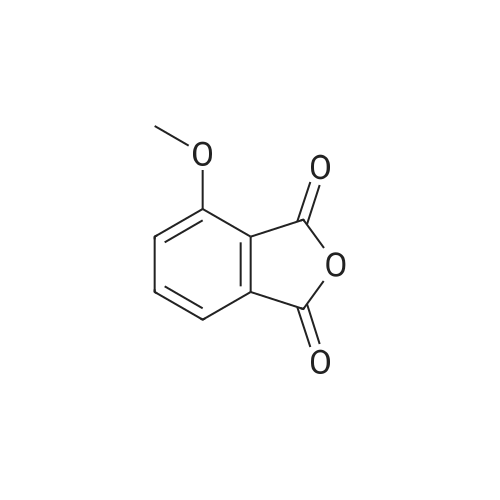
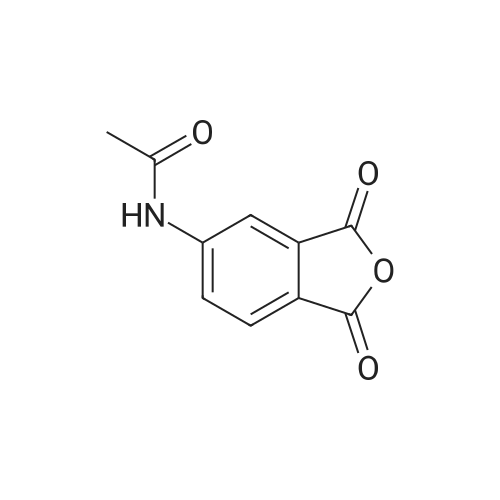
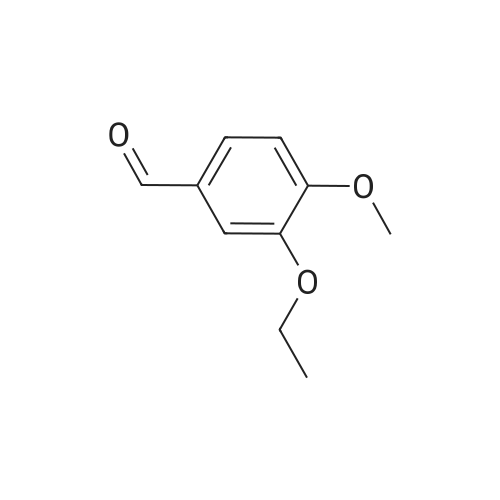
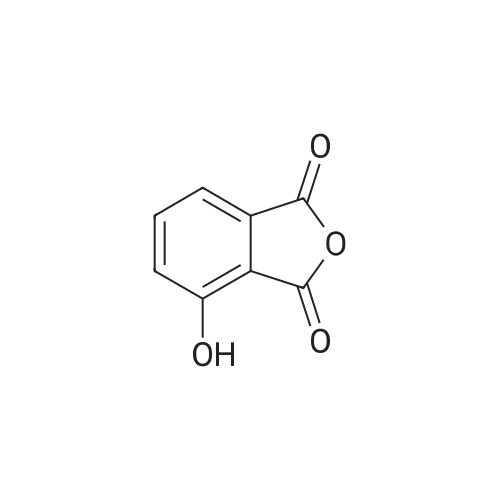
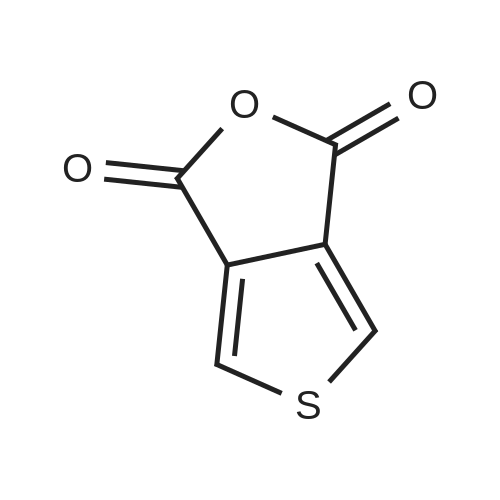
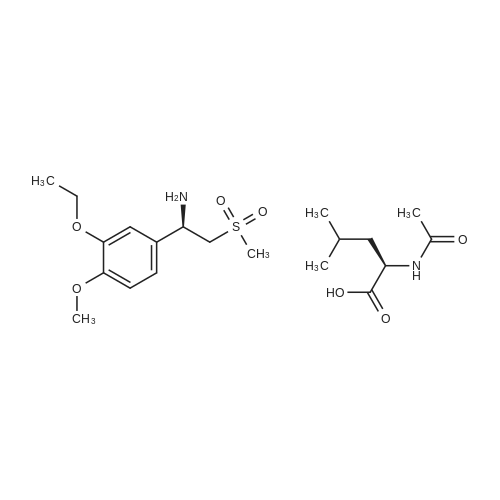
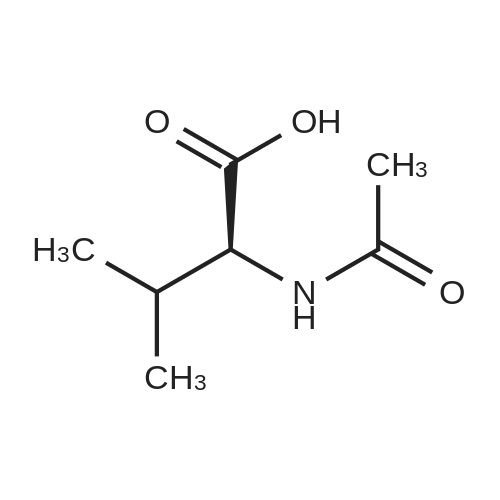
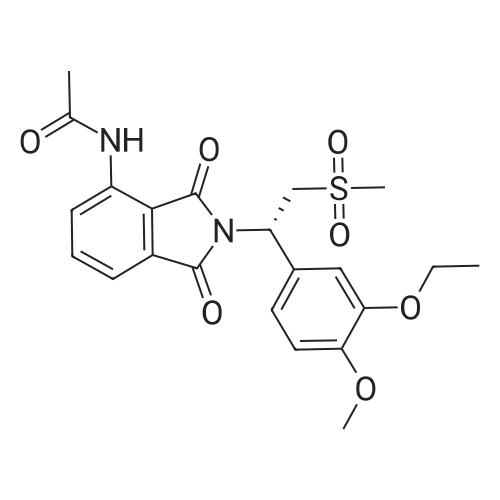
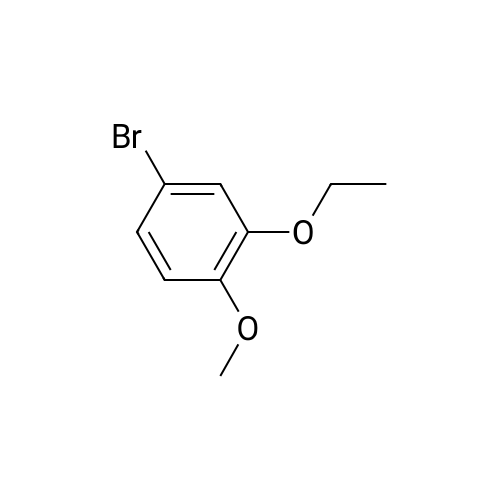
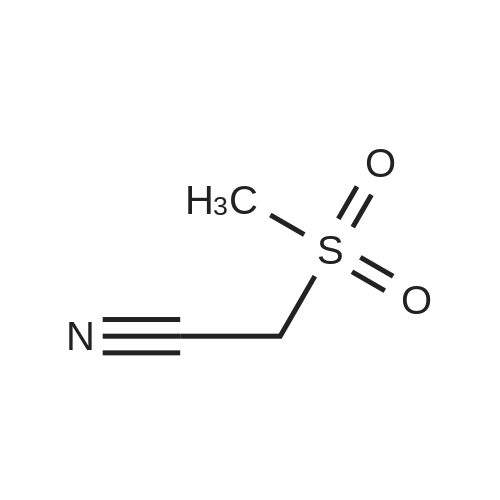
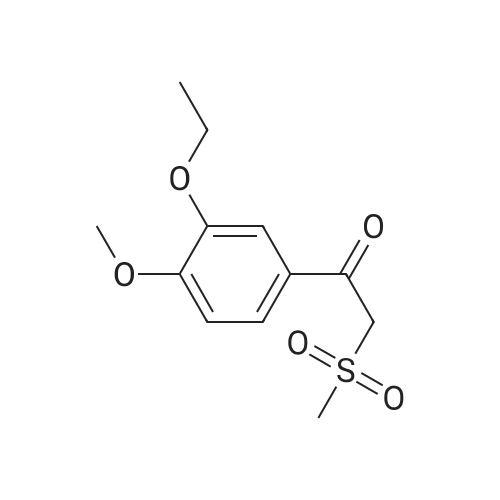
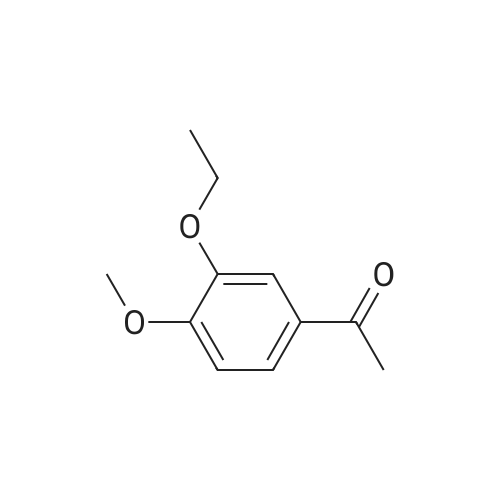
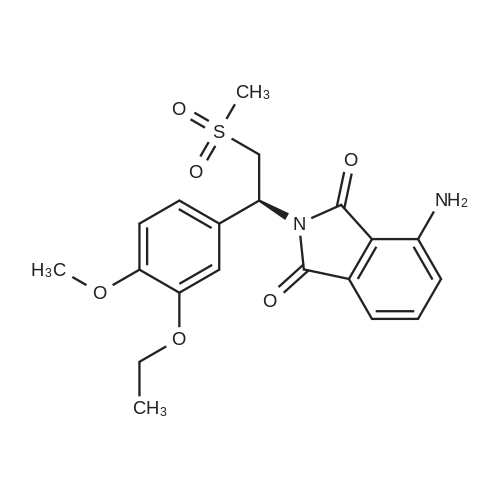
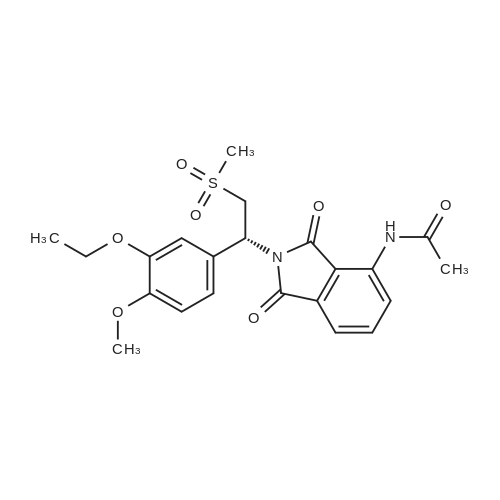
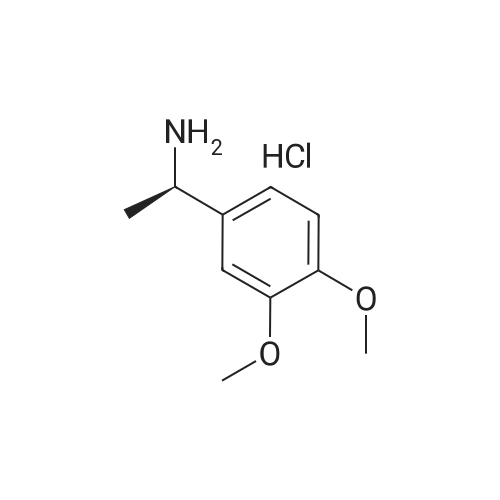
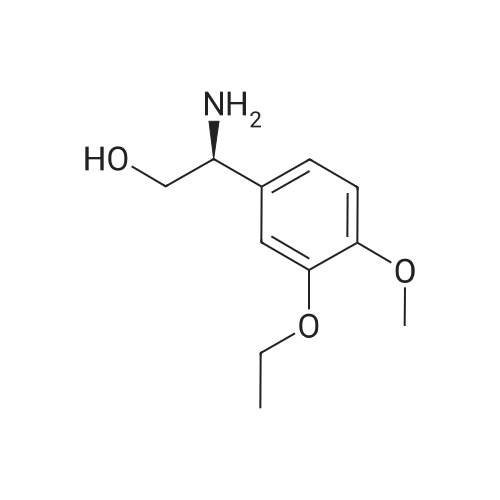
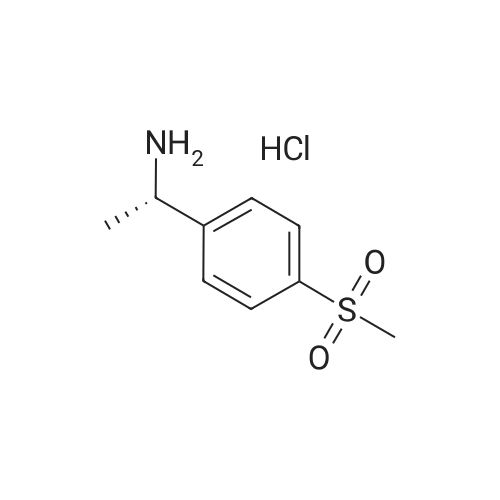
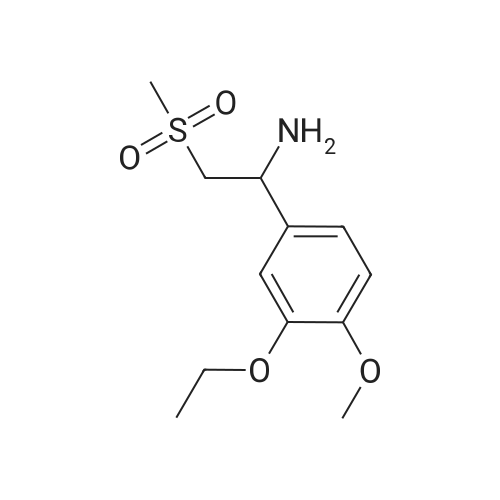
[0093] A stirred solution of l-(3-ethoxy-4-methoxyphenyl)-2- methylsulfonylethylamine (1.0 g, 3.7 mmol) and 3-acetamidophthalic anhydride (751 mg, 3.66 mmol) in acetic acid (20 mL) was heated at reflux for 15 h. The solvent was removed in vacuo to yield an oil. Chromatography of the resulting oil yielded the product as a yellow solid (1.0 g, 59% yield): mp, 144 C; 1H NMR (CDC13) delta: 1.47 (t, J=7.0 Hz, 3H, CH3), 2.26 (s, 3H, CH3), 2.88 (s, 3H, CH3), 3.75 (dd, J=4.4, 14.3 Hz, 1H, CH), 3.85 (s, 3H, CH3), 4.11 (q, J=7 Hz, 2H, CH2), 5.87 (dd, J=4.3, 10.5 Hz, 1H, NCH), 6.82-6.86 (m, 1H, Ar), 7.09-7.11 (m, 2H, Ar), 7.47 (d, J= 7 Hz, 1H, Ar), 7.64 (t, J= 8 Hz, 1H, Ar), 8.74 (d, J= 8 Hz, 1H, Ar), 9.49 (br s, 1H, NH); 13C NMR (CDC13) delta: 14.61, 24.85, 41.54, 48.44, 54.34, 55.85, 64.43, 111.37, 112.34, 115.04, 118.11, 120.21, 124.85, 129.17, 130.96, 136.01, 137.52, 148.54, 149.65, 167.38, 169.09, 169.40; Anal Calc'd. for C22H24NO7S : C, 57.38; H, 5.25; N, 6.08. Found: C, 57.31; H, 5.34; N, 5.83. |
| 59% |
With acetic acid; for 15h;Reflux; |
Example 1 Synthesis of 2-[1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl]-4-acetylaminoisoindoline-1,3-dione A stirred solution of 1-(3-ethoxy-4-methoxyphenyl)-methylsulfonylethylamine (1.0 g, 3.7 mmol) and 3-acetamidophthalic anhydride (751 mg, 3.66 mmol) in acetic acid (20 mL) was heated at reflux for 15 h. The solvent was removed in vacuo to yield an oil. Chromatography of the resulting oil yielded the product as a yellow solid (1.0 g, 59% yield): mp, 144 C.; 1H NMR (CDCl3) delta1.47 (t, J=7.0 Hz, 3H, CH3), 2.26 (s, 3H, CH3), 2.88 (s, 3H, CH3), 3.75 (dd, J=4.4, 14.3 Hz, 1H, CHH), 3.85 (s, 3H, CH3), 4.11 (q, J=7 Hz, 2H, CH2), 5.87 (dd, J=4.3, 10.5 Hz, 11-1, NCH), 6.82-6.86 (m, 1H, Ar), 7.09-7.11 (m, 2H, Ar), 7.47 (d, J=7 Hz, 1H, Ar), 7.64 (t, J=8 Hz, 1H, Ar), 8.74 (d, J=8 Hz, 1H, Ar), 9.49 (br s, 1H, NH); 13C NMR (CDCl3) delta14.61, 24.85, 41.54, 48.44, 54.34, 55.85, 64.43, 111.37, 112.34, 115.04, 118.11, 120.21, 124.85, 129.17, 130.96, 136.01, 137.52, 148.54, 149.65, 167.38, 169.09, 169.40; Anal Calc'd. for C22H24NO7S: C, 57.38; H, 5.25; N, 6.08. Found: C, 57.31; H, 5.34; N, 5.83. |
| 59% |
With acetic acid; for 15h;Reflux; |
A stirred solution of 1-(3-ethoxy-4-methoxyphenyl)-methylsulfonylethylamine (0607) (1.0 g, 3.7 mmol) and 3-acetamidophthalic anhydride (751 mg, 3.66 mmol) in acetic acid (20 mL) was heated at reflux for 15 h. The solvent was removed in vacuo to yield an oil. (0608) Chromatography of the resulting oil yielded the product as a yellow solid (1.0 g, 59% yield): mp, 144C; 1H NMR (CDC13) 51.47 (t, J=7.0 Hz, 3H, CH3), 2.26 (s, 3H, CH3), 2.88 (s,3H, CH3), 3.75 (dd, J=4.4, 14.3 Hz, 1H, CHH), 3.85 (s, 3H, CH3), 4.11 (q, J= 7 Hz, 2H, CH2), 5.87 (dd, J=4.3, 10.5 Hz, 1H, NCH), 6.82-6.86 (m, 1H, Ar), 7.09-7.11 (m, 2H, Ar), 7.47 (d, J= 7 Hz, 1H., Ar), 7.64 (t, J= 8 Hz, 1H, Ar), 8.74 (d, J= 8 Hz, 1H, Ar), 9.49 (br s, 1H, NH); 13C NMR (CDC13) 514.61, 24.85, 41.54, 48.44, 54.34, 55.85, 64.43, 111.37, 112.34, 115.04, 118.11, 120.21, 124.85, 129.17, 130.96, 136.01, 137.52, 148.54, 149.65, 167.38, 169.09, 169.40; Anal Calc'd. for C22H24NO7S: C, 57.38; H, 5.25; N, 6.08. Found: C, 57.31; H, 5.34; N, 5.83. |
| 59% |
With acetic acid; for 15h;Reflux; |
10213] A stirred solution of 1-(3-ethoxy-4-methoxyphe- nyl)-2-methyl sulfonylethylamine (1.0 g, 3.7 mmol) and 3-acetamidophthalic anhydride (751 mg, 3.66 mmol) in acetic acid (20 mL) was heated at reflux for 15 h. The solvent was removed in vacuo to yield an oil. Chromatography of the resulting oil yielded the product as a yellow solid (1.0 g, 59% yield): mp, 144 C.; ?H NMR (CDC13) oe: 1.47 (t, J=7.0 Hz, 3H, CH3), 2.26 (s, 3H, CH3), 2.88 (s, 3H, CH3), 3.75 (dd, J=4.4, 14.3 Hz, 1H, CH), 3.85 (s, 3H, CH3), 4.11 (q, J=7 Hz, 2H, CH2), 5.87 (dd, J=4.3, 10.5 Hz, 1H, NCH), 6.82-6.86 (m, 1H, Ar), 7.09-7.11 (m, 2H, Ar), 7.47 (d, J=7 Hz, 1H, Ar), 7.64 (t, J=8 Hz, 1H, Ar), 8.74 (d, J=8 Hz, 1H, Ar), 9.49 (br s, 1H, NH); ?3CNMR (CDC13) oe: 14.61,24.85,41.54,48.44,54.34, 55.85, 64.43, 111.37, 112.34, 115.04, 118.11, 120.21, 124.85, 129.17, 130.96, 136.01, 137.52, 148.54, 149.65, 167.38,169.09, 169.40; Anal Calc?d. for C22H24N075: C, 57.38; H, 5.25; N, 6.08. Found: C, 57.31; H, 5.34; N, 5.83. |
| 59% |
With acetic acid; for 15h;Reflux; |
A stirred solution of 1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethylamine (1.0 g, 3.7 mmol) and 3-acetamidophthalic anhydride (751 mg, 3.66 mmol) in acetic acid (20 mL) was heated at reflux for 15 h. The solvent was removed in vacuo to yield an oil. Chromatography of the resulting oil yielded the product as a yellow solid (1.0 g, 59% yield): mp, 144 C; 1H NMR (CDCl3) delta: 1.47 (t, J=7.0 Hz, 3H, CH3), 2.26 (s, 3H, CH3), 2.88 (s, 3H, CH3), 3.75 (dd, J=4.4, 14.3 Hz, 1H, CH), 3.85 (s, 3H, CH3), 4.11 (q, J=7 Hz, 2H, CH2), 5.87 (dd, J=4.3, 10.5 Hz, 1H, NCH), 6.82-6.86 (m, 1H, Ar), 7.09-7.11 (m, 2H, Ar), 7.47 (d, J= 7 Hz, 1H, Ar), 7.64 (t, J= 8 Hz, 1H, Ar), 8.74 (d, J= 8 Hz, 1H, Ar), 9.49 (br s, 1H, NH); 13C NMR (CDCl3) delta: 14.61, 24.85, 41.54, 48.44, 54.34, 55.85, 64.43, 111.37, 112.34, 115.04, 118.11, 120.21, 124.85, 129.17, 130.96, 136.01, 137.52, 148.54, 149.65, 167.38, 169.09, 169.40; Anal Calc'd. for C22H24NO7S: C, 57.38; H, 5.25; N, 6.08. Found: C, 57.31; H, 5.34; N, 5.83. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping