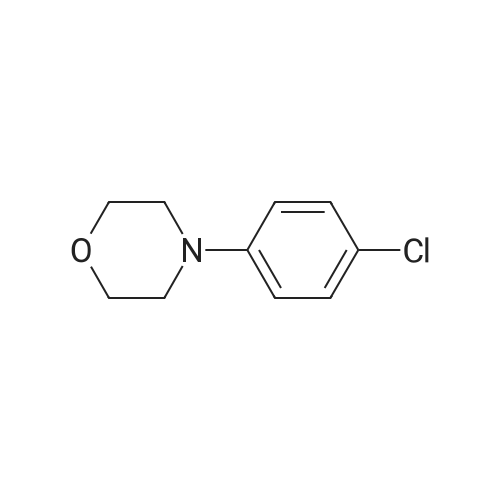
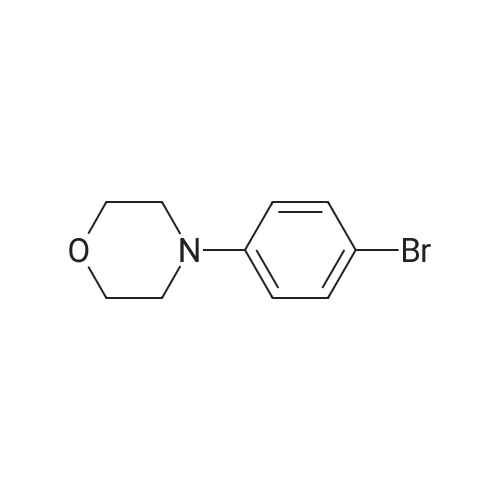
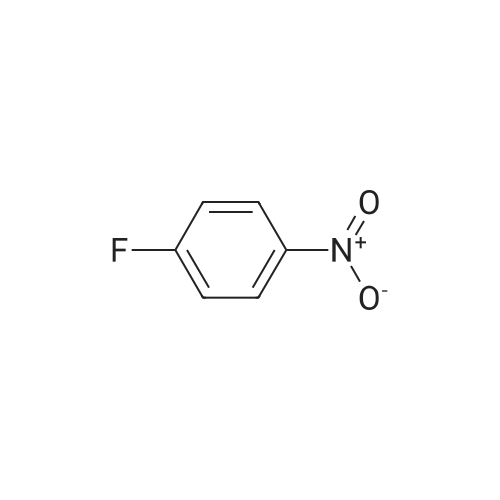
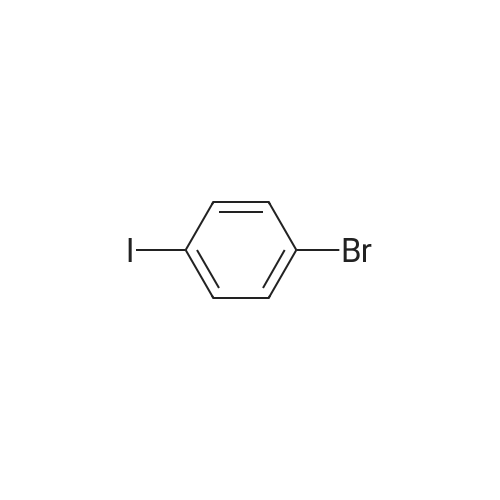
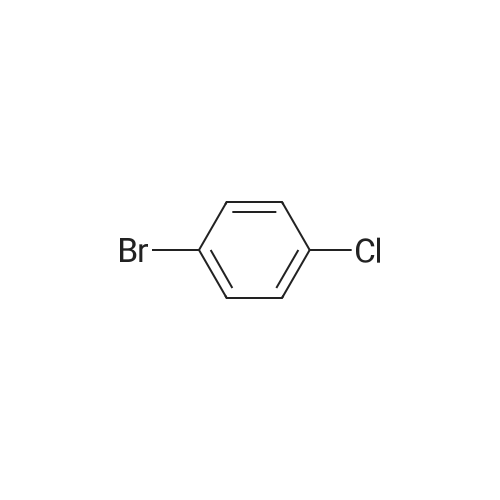
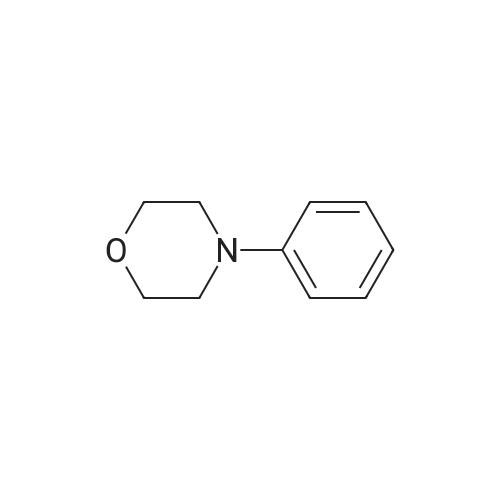
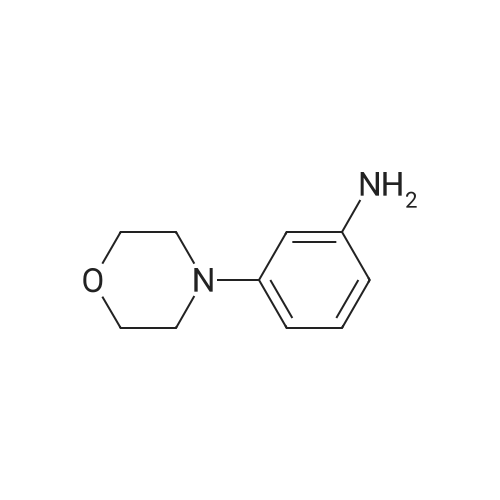
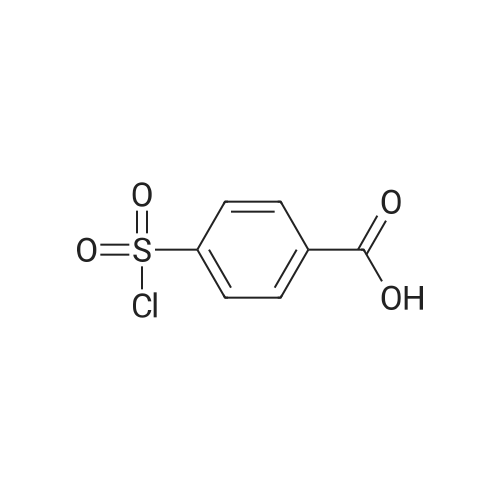
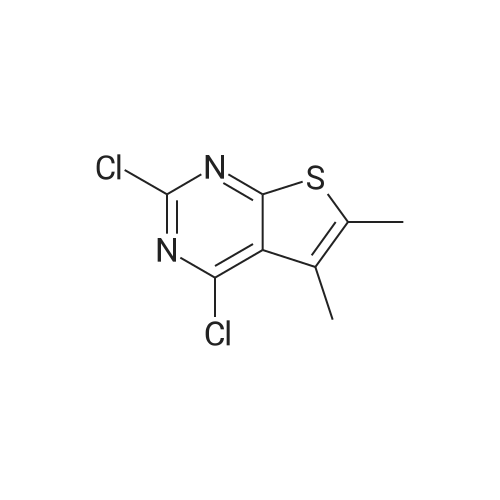
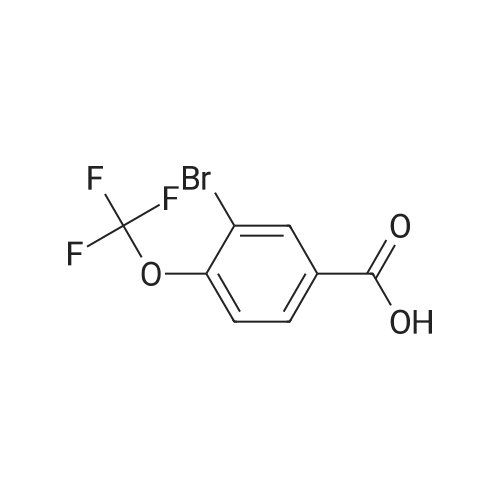
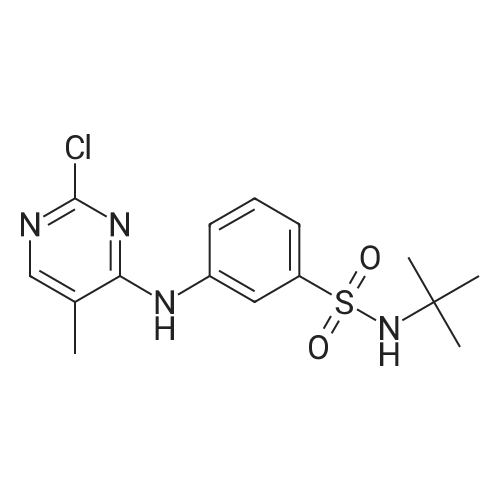
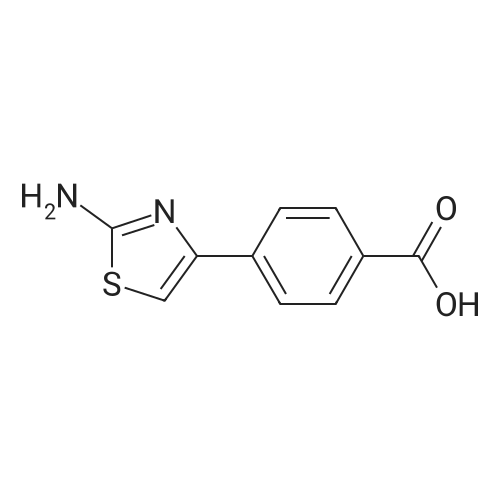
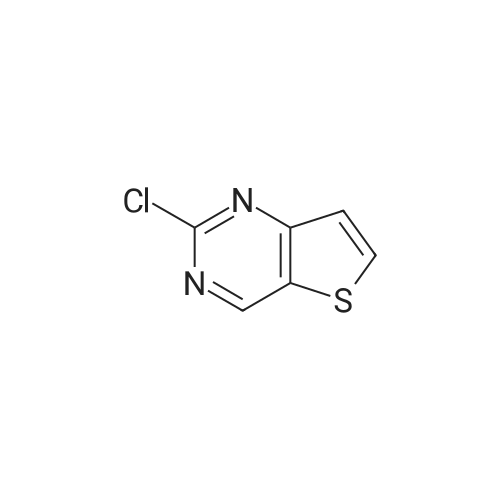
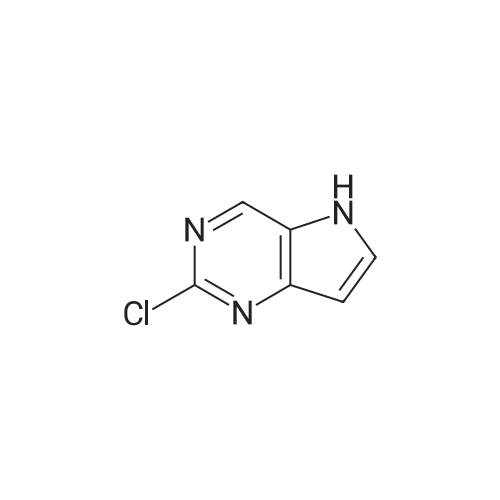
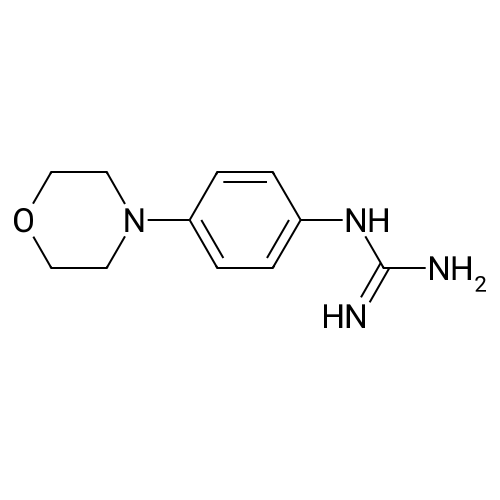
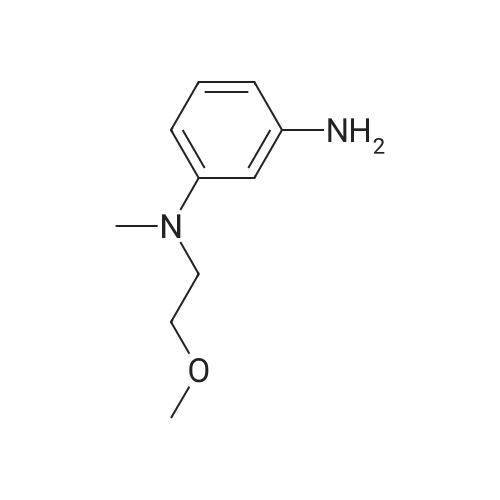
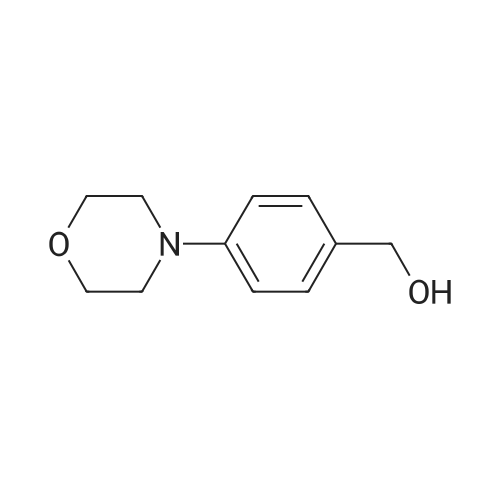
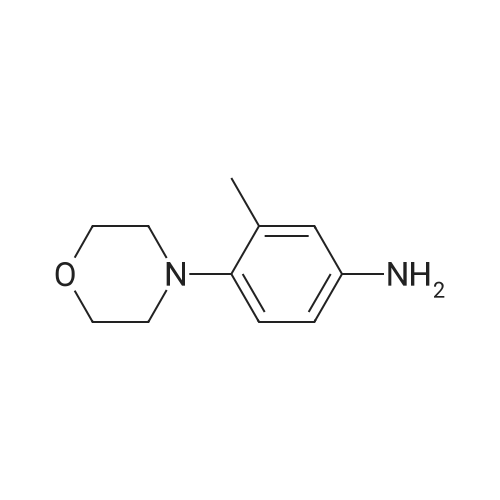
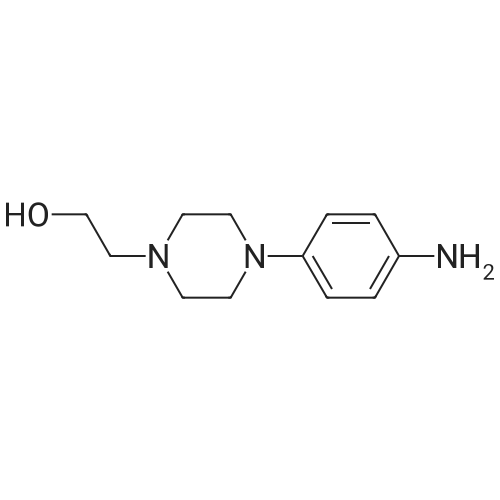
| 97% |
With palladium 10% on activated carbon; hydrogen; In methanol; at 40℃; for 18h; |
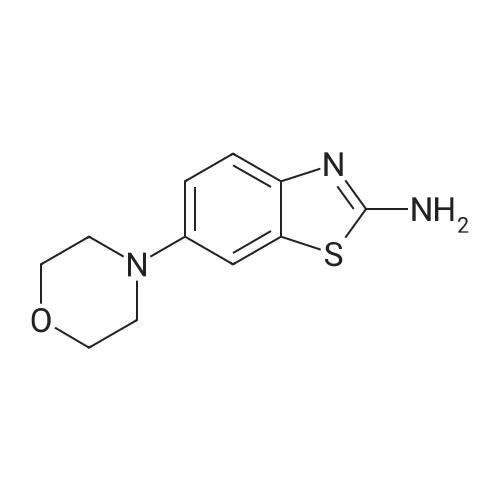
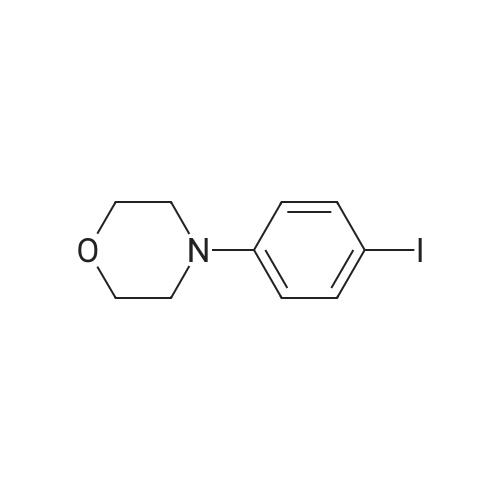
4-(4-nitrophenyl) morpholine (0.600 g, 2.882 mmol) and methanol (60 ml) were added to the hydrogenation reaction vessel. 10% Palladium on carbon (0.060 g) was added under nitrogen protection. The reaction system was replaced with hydrogen for 3-4 times, and was stirred under hydrogen pressure to react for 18 hours under 40C. HPLC detected that the reaction has completed, and then the reaction solution was cooled to room temperature, and filtered through Celite. The residue was washed with methanol. The combined filtrate was concentrated under vacumm by rotary evaporator to give the solid desired product (0.500 g, HPLC purity: 97.1%, yield: 97%). 1H NMR(400MHz, DMSO-d6) delta 6.83-6.80(2H, d), 6.71-6.68(2H, d), 3.88-3.86(4H, t), 3.46(2H, s), 3.06-3.03 ppm(4H, t). |
| 97% |
With palladium on activated charcoal; hydrogen; In methanol; at 20℃; |
General procedure: Afterwards, the obtained compound, 2a was hydrogenated. Pd/C (2.78mmol) was added to a stirred solution of 1-methyl-4-(2-methyl-4-nitrophenyl-piperazine (5.53mmol)) in methyl alcohol (15ml) in N2 atmosphere. Following this it was hydrogenated overnight by maintaining the reaction flask in an atmosphere of H2 gas (balloon). The resultant was filtered thorough celite plate and concentrated under vacuum. The crude product was purified with silica-gel column chromatography (5%MeOH/DCM, 1%NH3) to yield the desired product, 3a as a brown solid, 87% yield. |
| 97.4% |
|
11c (0.6 g, 2.9 mmol) was dissolved in tetrahydrofuran (12.5 ml), and methanol (12.5 ml) was added and stirred for 5 min. Ammonium chloride (1.5 g, 28.8 mmol) in water (6.5 mL) was added and the mixture was continued to stir for 30 min. Zinc powder (942 mg, 14.4 mmol) was added to the system and the reaction was continued to stir at 17 C for 3 hours. The reaction liquid was gradually changed from bright yellow to gray, and the reaction was monitored to have been finished by thin-plate chromatography (methanol / dichloromethane = 1:10). After stood for 15 minutes, the reaction was filtered through celite, the cake was rinsed with ethyl acetate (10.5 ml) and the filtrate was concentrated to dryness under reduced pressure. Water was added (5.5 ml) and extracted with ethyl acetate (10 ml x 3). The combined organic phase was washed with saturated sodium chloride solution (10.2 mL), dried and concentrated under reduced pressure to give a pale yellow solid 11d (500 mg, 99.1% purity, yield 97.4%). |
| 96% |
With palladium 10% on activated carbon; hydrogen; In ethanol; at 20℃; for 5h; |
26.9 g (129 mmol) of Intermediate B and 1.34 g (5% m/m) of 10% palladium on carbon were added to 500 mL of a 90% ethanol solution, and hydrogen gas was introduced thereto, and reacted at room temperature for 5 hours.After the reaction was completed, suction filtration, washing the filter cake with 100 mL of 90% ethanol.The filtrate was concentrated under reduced pressure to give a purple solid (22.0 g).The yield was 96%. |
| 95% |
With palladium 10% on activated carbon; hydrazine hydrate; In ethanol; at 75℃; for 8h; |
General procedure: 10% Pd/C(1.43 g, 13.5 mmol), hydrazine (8 mL) and nitrobenzene derivatives2a-2c and 4a-4d (19 mmol) were dissolved in ethanol (60 mL) and useargon to remove the gas three times. The resulted mixture was heated to75 C for 8 h. After that, the reaction solution was filtered throughcelite. Then the crude products were purified by column chromatographyon silica gel to afford desired intermediates 3a-3b, 5a-5e. |
| 94.5% |
With hydrogen; In tetrahydrofuran; at 20℃; under 760.051 Torr; for 10h; |
4-(4-nitrophenyl)morpholine (8.33 g, 0.04 mol) was added to a 250 mL eggplant-shaped flask, 100 mL of tetrahydrofuran was added, and the mixture was stirred at room temperature, and 6 g of Raney nickel was added.After vacuuming for 3 times, hydrogen gas was passed through a hydrogen balloon (pressure was 1 atm), and the reaction was carried out at room temperature. The reaction was followed by thin layer chromatography (TLC). After 10 hours, TLC showed the reaction was finished, and the reaction mixture was filtered over Celite, and tetrahydrofuran (20 mL) ×2) Washing diatomaceous earth,The tetrahydrofuran was evaporated to dryness to give 7.91 g of the white solid, 4-morpholinylaniline, yield 94.5% |
| 87% |
With palladium on activated charcoal; hydrogen; In methanol; at 20℃; |
4-Fluoronitrobenzene (382 mg, 2.7 mmol) was dissolved in DMSO (7 ml), potassium carbonate (561 mg, 4.0 mmol) and morpholine (472 mg, 5.4 mmol) were added, and the mixture was stirred at 90 C. overnight. Then, water was added to the reaction solution, and the mixture was extracted twice with ethyl acetate. The organic layer was washed twice with saturated aqueous NaCl. The organic layer was dried over Na2CO3, the solvent was evaporated, and the obtained residue was purified by silica gel chromatography (eluent; hexane:ethyl acetate (2:1)) to give compound Y180 (yield; 493 mg, 88%). Compound Y180 (483 mg, 2.3 mmol) was dissolved in methanol (25 ml), Pd/C (205 mg) was added, and the mixture was stirred under a hydrogen atmosphere at room temperature overnight. Then, the reaction solution was filtered through celite, the filtrate was concentrated under reduced pressure, and the obtained residue was purified by silica gel chromatography (eluent; hexane:ethyl acetate (1:2)) to give compound Y183 (yield; 358 mg, 87%). Compound Y491 (mentioned later) (134 mg, 0.3 mmol) was dissolved in dichloromethane (4 ml), compound Y183 (158 mg, 0.9 mmol) and triethylamine (123 mul, 0.9 mmol) were added, and the mixture was stirred at room temperature for 45 min. Then, the solvent was concentrated under reduced pressure, and the obtained residue was purified by silica gel chromatography (eluent; chloroform:methanol (22:1)) to give the title compound (yield; 120 mg, 69%). 1H NMR (500 MHz, CDCl3) delta8.43 (s, 1H), 8.00 (d, 1H, J=8.0 Hz), 7.70 (d, 1H, J=8.0 Hz), 7.53 (t, 1H, J=8.0 Hz), 7.43 (bs, 1H), 6.98 (dd, 2H, J=9.0, 3.5 Hz), 6.76 (dd, 2H, J=9.0, 3.5 Hz), 5.55 (dd, 1H, J=6.5, 6.0 Hz), 4.05 (bs, 2H), 3.83-3.81 (m, 4H), 3.10-3.08 (m, 4H), 2.80-2.63 (m, 4H), 1.64-1.54 (m, 3H), 1.42 (s, 9H), 1.86-1.01 (m, 2H) 13C NMR (125 MHz, CDCl3) delta155.0, 150.1, 141.6, 140.8, 131.3, 131.0, 129.8, 127.4, 125.8, 125.6, 116.2, 79.7, 66.9, 58.6, 50.9, 49.2, 48.7, 36.5, 29.6, 28.6, 18.5 HRMS (FAB-) m/z: [M-H]- calcd for C27H37N4O7S2, 593.2104. found, 593.2197 |
| 87.4% |
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; for 17h;Inert atmosphere; |
4-(4-Nitrophenyl)morpholine (2.20 g, 10.6 mmol) was dissolved in 12 mL of methanol.Replace the nitrogen,Add 30mg of 10% palladium on carbon,The system replaces hydrogen three times,The reaction was stirred at room temperature for 17 h.Palladium charcoal is filtered off,The filtrate was dried to give a pale yellow oil, 1.65 g.Yield: 87.4%. |
| 86% |
With ammonium chloride; zinc; In ethanol; water; for 0.5h;Reflux; |
Compound 7-b (1.5 g, 7.21 mmol) and ammonium chloride (1.0 g, 18.03 mmol) were dissolved in 50% ethanol-water (20 mL), Zn-powder (1.2 g, 18.03 mmol) was then added. The mixture was refluxed for 30 minutes. After cooled to room temperature, the mixture was filtrated. The filter cake was washed with ethanol (10 mL), the combined filtrate were concentrated under reduced pressure. The residue was diluted with water (50 mL) and extracted with ethyl acetate (50 mL×3), the organic layer was dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to give light brown solid 7-a (1.1 g, yield: 86%), which was used directly for the next step without purification. LC-MS (ESI): m/z=179 [M+H]+. |
| 85% |
With palladium 10% on activated carbon; hydrogen; In ethyl acetate; at 80℃; under 760.051 Torr; for 12h; |
To a mixture of 5a (2.13g, 10mmol) in dry ethyl acetate was added 10% Pd/C (0.5g), following the reaction mixture was stirred under hydrogen gas at 80C for 12h. The reaction mixture was concentrated in vacuo and the crude product was purified by column chromatography (dichloromethane/methanol 15:1 by volume) to give 4-morpholinyl aniline 6a (1.5g, yield 85%). 1H NMR (400MHz, CDCl3) delta 6.81-6.77 (m, 2H), 6.68-6.64 (m, 2H), 3.85-3.83 (m, 4H), 3.03-3.01 (m, 4H). |
| 85% |
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; |
4-(4-nitrophenyl)morpholine (1 g, 4.80 mmol) and methanol (30 mL) were placed in a reaction vessel. 10% Pd / C (100 mg) was added to the mixed solution, and the mixture was stirred at room temperature under a balloon pressure filled with hydrogen gas. The reaction solution was filtered through a pad of diatomaceous earth and concentrated. The target compound (728 mg, 85% yield) was used in the next reaction without purification. |
| 77% |
With water; ammonium chloride; zinc; In tetrahydrofuran; methanol; at 20℃; |
Synthesis of 4-Morpholin-4-yl-phenylamine Ammonium chloride (1.2 g, 23.0 mmol) in water (4.5 mL) then MeOH (9 mL) were added to a stirred solution of 4-(4-nitro-phenyl)-morpholine (480 mg, 2.3 mmol) in THF (9 mL). Zinc powder (1.2 g, 18.4 mmol) was then added portion wise and the resulting mixture was stirred at room temperature for 15 minutes. The reaction mixture was filtered over celite and the filtrate was extracted with ethyl acetate. The organic layer was washed with brine, dried over Na2SO4 and concentrated to afford 316 mg (77%) of 4-morpholin-4-yl-phenylamine. 1H NMR: (DMSO-d6): delta 6.7 (m, 2H), 6.5 (m, 2H), 4.6 (s, 2H), 3.7 (t, 4H), 2.8 (t, 4H). |
| 74.1% |
With iron(III) chloride hexahydrate; pyrographite; hydrazine hydrate; In ethanol; at 65 - 78℃; for 5h; |
General procedure: A mixture of compounds 5a-e (0.05 mol) in ethanol was heated to 65 C, FeCl3·6H2O (2.8 g, 0.001 mol) and activated carbon (0.18 g, 0.015 mol) were added, and 80% hydrazine hydrate (25 g, 0.5 mol) was added drop wise at such a rate to keep the temperature below 70 C, the reaction was heated at reflux for 5 h and then cooled to room temperature and concentrated. Water (100 mL) was added, the reaction solution was extracted three times with DCM. The organic extracts were combined, dried over sodium sulfate, filtered, and concentrated to obtain the compounds 6a-e. |
| 74.1% |
With iron(III) chloride hexahydrate; hydrazine hydrate; In ethanol; for 5h; |
General procedure: A mixture of compounds 5 or 7a-c (0.05 mol) in ethanolwas heated to 65 C, FeCl3·6H2O (2.8 g, 1 mmol) and activatedcarbon (0.18 g, 15 mmol) were added, and 80% hydrazinehydrate (25 g, 0.5 mol) was added dropwise at such arate to keep the temperature below 70 C, the reaction washeated at reflux for 5 h and then cooled to room temperatureand concentrated. Water (100 mL) was added, the reactionsolution was extracted three times with CH2Cl2 (60 mL). Theorganic extracts were combined, dried over sodium sulfate,filtered, and concentrated to obtain the compounds 8a-d. |
| 74% |
With palladium 10% on activated carbon; hydrogen; In ethanol; at 20℃; for 23h; |
To a solution of 4-(4-nitrophenyl)morpholine(8.45 g, 0.041 mol) in EtOH (250 mL) was added 10% Pd/C(0.843 g, 10% w/w). The resulting mixture was stirred at roomtemperature under an atmosphere of hydrogen for 23 h. Aftercompletion, the mixture was filtered through Celite and washedwith EtOH. The solvent was removed in vacuo to give the titlecompound 8 (5.25 g, 74%) as a pink solid which was used withoutfurther purification. m.p. 129-130 C (Lit [21]. 129.5-130.5 C). deltaH(400 MHz; CDCl3) 3.02 (4H, t, J 4.7 Hz, H-30), 3.44 (2H, s, NH2),3.85, (4H, t, J 4.7 Hz, H-20), 6.66, (2H, d, J 8.8 Hz, H-3), 6.80 (2H,d, J 8.8 Hz, H-2). deltaC (100 MHz; CDCl3) 51.1 (C-30), 67.0 (C-20), 116.2(C-2), 118.1 (C-3), 140.3 (C-1), 144.4 (C-4); IR numax(ATR)/cm-1: 3393,2855, 1641, 1411, 1259, 1107, 825; m/z (ESI+): 227 (17%), 179 (MH,100%); HRMS (ESI+) found (M+H): 179.1178, C10H15N2O requires179.1179. The 1H NMR data were in agreement with literaturevalues [22]. |
| 73% |
With iron; acetic acid; In methanol; water; |
4 g of iron powder, 30 mL of water and 0.5 mL of glacial acetic acid were added to a 100 mL round bottom flask and heated to reflux for 10 min.Then, a solution of 3.12 g (0.015 mol) of 4-(4-nitrophenyl)morpholine dissolved in 20 mL of methanol was added, the reaction was stirred, and the reaction was traced to the end point by TLC.A small amount of anhydrous sodium sulfite was added, and after cooling, the pH was adjusted to 8-9 with a 10% sodium carbonate solution, and filtered through Celite.The filter cake was washed with hot ethanol, and the filtrate was evaporated to a solvent.Washed with saturated sodium carbonate solution and saturated sodium chloride solution, the organic phase was dried over anhydrous sodium sulfate, and concentrated to give a yellow solid 1.96g,Melting point: 132-133 C, yield 73%. |
| 73% |
With iron; acetic acid; In methanol; water; |
Weigh 4g of iron powder in a 100ml round bottom flask.Add 30 ml of water and 0.5 ml of glacial acetic acid.Activated under reflux for 10 min,Then weighed 3.12 g (0.015 mol) of the previous synthesis4-(4-nitrophenyl)morpholine in 20 ml of methanol,Add to the reaction bottle,TLC tracks the reaction, the reaction is complete,Add a small amount of anhydrous sodium sulfite, cool and then adjust the pH to 8-9 with 10% sodium carbonate solution, filter with diatomaceous earth, filter cake with hot ethanol, until the filtrate is colorless, the filtrate is steamed a part of solvent After that, it was extracted with ethyl acetate, washed successively with saturated sodium carbonate solution and saturated sodium chloride solution, and the organic phase was dried over anhydrous sodium sulfate.Concentrated to give 1.96 g of a yellow solid.The yield was 73%. |
| 70% |
With ammonia;palladium; In methanol; |
REFERENCE EXAMPLE 20 Preparation of 4-Morpholin-4-yl-phenylamine 4-(4-Nitrophenyl)morpholine (10.3 g, 49.5 mmol;) (Lancaster Synthesis) was suspended in methanol (130 ml) and 2 M ammonia in methanol (70 mL) and 5% palladium on carbon (100 mg) was added. The mixture was hydrogenated on a Paar apparatus (50 psi) for 1 h. The reaction was allowed to cool, the catalyst was filtered and the solution was concentrated in vacuo. The crude solid was recrystallized from ethyl acetate/hexane to give 4-(4-morpholinyl)aniline as a light purple solid (6.2 g, 70% yield, mp 132-133 C.). GC/CMS (EI, M+) m/z=178. |
| 70% |
With ammonia;palladium; In methanol; |
REFERENCE EXAMPLE 20 Preparation of 4Morpholin-4-yl-phenylamine. 4(4-Nitrophenyl)morpholine (10.3 g, 49.5 mmol;). (Lancaster Synthesis) was suspended in methanol (130 ml) and 2 M ammonia in methanol (70 mL) and 5% palladium on carbon (100 mg) was added. The mixture was hydrogenated on a Paar apparatus (50 psi) for 1 h. The reaction was allowed to cool, the catalyst was filtered and the solution was concentrated in vacuo. The crude solid was recrystallized from ethyl acetate/hexane to give 4-(4-morpholinyl)aniline as a light purple solid (6.2 g, 70% yield, mp 132-133 C.). GC/MS (EI, Mt) m/z=178. |
| 70% |
With ammonia;palladium; In methanol; |
Reference Example 20 Preparation of 4-Morpholin-4-yl-phenylamine 4-(4-Nitrophenyl)morpholine (10.3 g, 49.5 mmol;) (Lancaster Synthesis) was suspended in methanol (130 ml) and 2 M ammonia in methanol (70 mL) and 5% palladium on carbon (100 mg) was added. The mixture was hydrogenated on a Paar apparatus (50 psi) for 1 h. The reaction was allowed to cool, the catalyst was filtered and the solution was concentrated in vacuo. The crude solid was recrystallized from ethyl acetate/hexane to give 4-(4-morpholinyl)aniline as a light purple solid (6.2 g, 70% yield, mp 132-133 C.). GC/MS (EI, M+) m/z=178. |
| 68% |
With iron; ammonium chloride; In methanol; water; at 60℃; for 2h; |
10 (7.13 g, 34.26 mmol) and ammonium chloride (3.66 g, 68.52 mmol) were added to the reaction flask and 30 ml of MeOHAnd water (30 ml) was added with iron powder (7.67 g, 137.04 mmol) under stirring. The reaction was carried out at 60 C for 2 hours. The filter was washed with n-butanol and extracted with n-butanol (150 ml x 3) Washed with water, dried over anhydrous sodium sulfate and evaporated to dryness under reduced pressure to give 4.15 g of a dark brown solid (yield: 68%). |
|
With ammonium formate;palladium 10% on activated carbon; In tetrahydrofuran; methanol; at 20℃; for 7h; |
N-(4-nitrophenyl)morpholine (18.7g) and (400ml) ammonium formate (23.5g) were suspended in a mixture tetrahydrofuran-methanol: lvol-4vol (400ml) and 10% Pd/C (0.5g) was added to the stirred suspension by portion for 5min. The mixture was <n="13"/>stirred at ambient temperature for 3 hours. Then a mixture tetrahydrofuran-methanol: lvol-4vol (100ml) and ammonium formate (12g) were added to the reaction mixture at once, followed by addition in portions of 10% Pd/C (0.5g) with stirring. The reaction mixture was stirred for additional 4 hours. The solid was filtered, and the solvents from the mother liquor were evaporated under vacuum. The rest was triturated in a mixture ethyl acetate-water (80ml-100ml). The solid was filtered, dried (3O0C, vacuum 10mm, 8 hour) and gave 4-(4-morpholinyl)aniline (11.5g). |
|
With palladium on activated charcoal; hydrogen; In methanol; at 20℃; for 2h; |
General procedure: Nitro-compound (2a-2e) was dissolved in methanol and stirred at room temperature with palladium/charcoal (5mol%) in a hydrogen gas environment for 2h. The resulting solution was filtered through celite, concentrated, and used in the next step without further purification. |
|
With hydrazine; In ethanol;Reflux; |
General procedure: 4-Nitrobenzyl bromide (46.3mmol) was dissolved in dichloromethane (100mL). The solution was added to the mixture of relative amine (47.0mmol) and triethylamine (70.3mmol) in dichloromethane (20ml). The reaction mixture was stirred at r. t. for 24 h and was extracted with dichloromethane (100ml×3). After removal of the solvent, the residue was crystallized from ethanol, giving yellow powder. Compounds 1 and 2 were used for further reaction without purification. To a suspension of compounds 1-2 (36.2mmol) in 95% ethanol (100ml), 85% NH2NH2·H2O (362mmol), 95% ethanol (100ml) and iron (III) oxide hydroxide (FeO(OH)/C, 2.0g) were added and heated to reflux. When TLC analysis showed complete conversion of the starting material, the reaction mixture was filtrate through Cellit and the filtrate was concentrated in vacuum. The crude product was purified by silica gel colum chromatography (DCM/MeOH) to yield the title compound (3 and 4) as white solid. The mixture of compound 4 (1eq, 18.5mmol), 4-Nitro-1H-pyrazole-3-acid (1.1equiv, 20.4mmol), EDC (1.2equiv, 22.2mmol), HOBT (1.2equiv, 22.2mmol) in DMF (50ml) was stirred for 24h. The ice water (100ml) was added to the reaction mixture. A large amount of yellow solid precipitation (compound 8) was acquired. Compound 8 was used without further purification. Compounds 8 was reduced by the same process as compound 4, and then the resulting compound 12 was purified by column chromatography on silica gel, eluted with the appropriate solvent. |
| 209 mg |
With iron; ammonium chloride; In methanol; water; at 100℃; |
General procedure: The compound S1 (261 mg, 1.25 mmol), iron powder (210 mg, 4.76 mmol, 3 equiv.) and ammonium chloride (335 mg, 6.27 mmol, 5 equiv.) were dissolved in methanol : water (2 : 1, 15 mL). The reaction mixture was heated at 100 C overnight, cooled to RT, filtered through celite and the solvent was reduced under vacuum. The condensed mixture was extracted with DCM, washed with brine, dried with sodium sulfate and all solvent was evaporated to furnish the condensed residue, which was purified by flash chromatography (elution system - EA/Hexane = 1 : 1 ) to obtain the title compound (245 mg, 1.43 mmol). |
|
With 5%-palladium/activated carbon; hydrogen; In ethanol; water; at 20℃; |
General procedure: The substituted nitro compound 11 (1 equiv in a mixture of EtOH-H2O, 95:5, 20mL) was treated with 10% Pd-carbon (5% w/w). The reaction was subjected to hydrogenation under hydrogen gas at room temperature and the reaction was monitored by TLC. After completion of the reaction, the mixture was filtered through a Celite bed and concentrated in a vacuum to afford product 12. |
| 0.85 g |
With palladium on activated charcoal; hydrogen; In ethanol; at 20℃; for 24h; |
4-Fluoronitrobenzene (1 g), K2CO3 (1.1 g) and 5 ml DMSO were stirred at room temperature for 30 min.Add morpholine (0.6 g) dropwise, stir at 220 C for 2 h, pour the mixture into 20 mL of alcohol and water mixture (1:1).The yellow precipitate was filtered to give 4-(4-nitrophenyl)morpholine 1.4 g.4-(4-Nitrophenyl)morpholine (1 g), Pd/C (0.1 g) and 20 ml of ethanol were exchanged in a balloon of hydrogen at room temperature for about 24 h.The diatomaceous earth was filtered under reduced pressure, and the filtrate was dried under reduced pressure.The title product was obtained (0.85 g). |
| 2.2 g |
With palladium 10% on activated carbon; hydrogen; In ethanol; at 20℃; for 12h; |
PdeC (0.27 g, 10% m/m) wasadded to a solution of 8a (2.7 g, 13.0 mmol) in ethanol (20 ml) andhydrogenated for 12 h at room temperature. The resultant wasfiltered, washed with ethanol and concentrated. 2.2 g of 9a aspurple solid was obtained; yield: 94%; 1H NMR (600 MHz,DMSO-d6) delta 6.68 (d, J 8.5 Hz, 2H), 6.50 (d, J 8.5 Hz, 2H), 4.62 (s,2H), 3.69 (t, J 4.8 Hz,4H), 2.87 (t, J 4.8 Hz,4H). MS (ESI) m/z:179.2 [MH]. |
|
With FeO(OH)/C; hydrazine hydrate; In ethanol; at 0 - 80℃; for 4h; |
General procedure: To a solution of 1a (5 g, 22.5 mmol) in 95% ethanol (100 mL) wasadded goethite (FeO(OH))/C (1.0 g) at room temperature. And a solution of 80% hydrazine hydrate (25 mL, 400 mmol) in 95% ethanol (50 mL) was added dropwise to the mixture at 0 C. The reaction mixture was stirred at 80 C for 4 h. The solvent was removed in vacuo to give 2a (3.8 g, yield 74.5%). MS m/z: 278.2 [M+H]+. |
| 0.85 g |
With palladium on activated charcoal; hydrogen; at 10 - 35℃; for 24h; |
Add 4-fluoronitrobenzene (1g), K2CO3 (1.1g) and 5ml DMSO,The reaction was stirred at room temperature for 30 min, and morpholine (0.6 g) was added dropwise.Heat the reaction at 120 C for 2h and pour the mixture into 20mL of alcohol and water mixed solution (1: 1).The yellow precipitate was filtered to give 1.4 g of 4- (4-nitrophenyl) morpholine.Add 4- (4-nitrophenyl) morpholine (1g), Pd / C (0.1g) and 20ml ethanol,The air was replaced with a hydrogen balloon, and the reaction was performed at room temperature for about 24 hours. Diatomite vacuum filtration,The filtrate was dried under reduced pressure to obtain the title product.(0.85g). |
|
With sodium tetrahydroborate; In water; for 1h; |
General procedure: The catalytic activities of Au/TNT and Au/ANT for the reduction of4NP with NaBH4 were evaluated using 0.015 g of catalyst samples andthe necessary amounts of substrate to obtain a molsubstrate;molAu ratio of500, based on the metal contents determined by AAS. The experimentalprocedure was measured employing a glass reactor (500 mL) equippedwith a mechanical stirrer (Figure S2). All activity performances weredetermined in triplicate. Initially, DI water (17.2 mL), the catalyst(0.015 g), and the 4NP solution (3.8 mL, 0.05 M) were mixed in thereactor at 400 rpm for 15 min. Subsequently, a freshly prepared 1.0Maqueous NaBH4 solution (19 mL) was added, and this was considered asthe zero time. The catalytic reduction of 4NP was then monitored bytaking non-invasive samples (500 ]L) periodically from the reactionmixture at different reaction times, and the reaction progress wasmonitored by UV-vis spectrophotometry between 250 and 550 nm. Toconfirm the obtained results, after each reaction, the samples wereanalyzed using the HPLC-DIONEX liquid chromatography system. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping