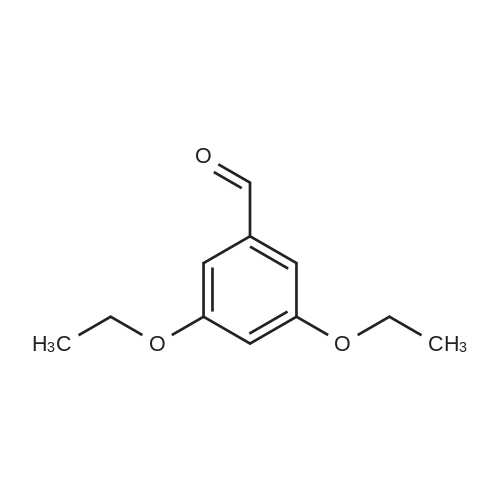
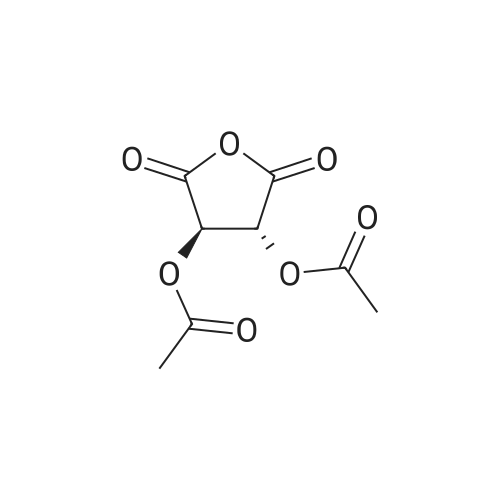
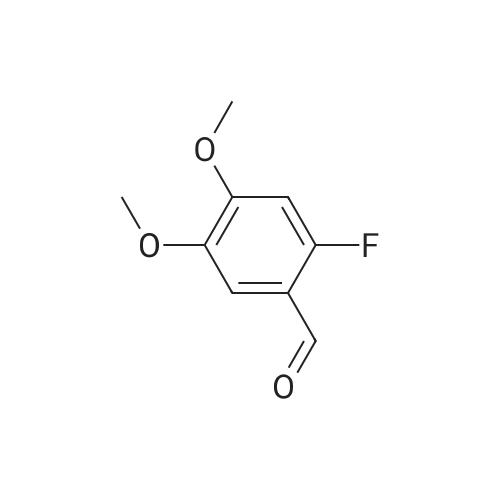
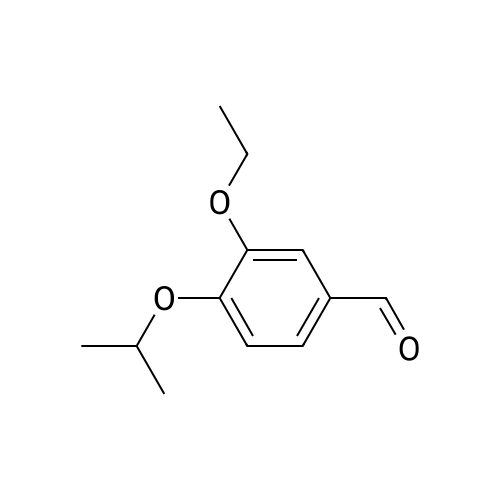
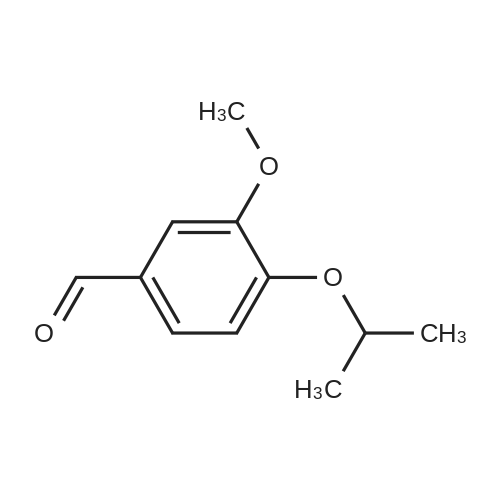
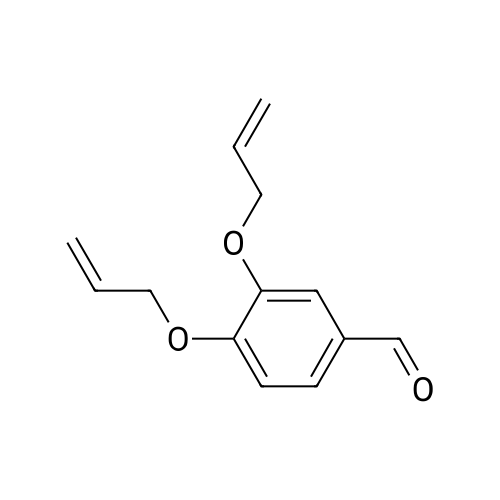
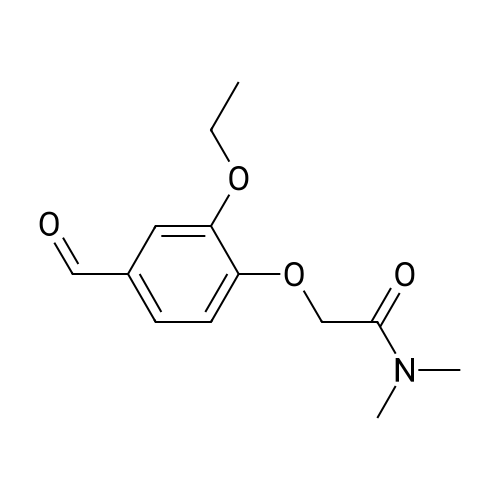
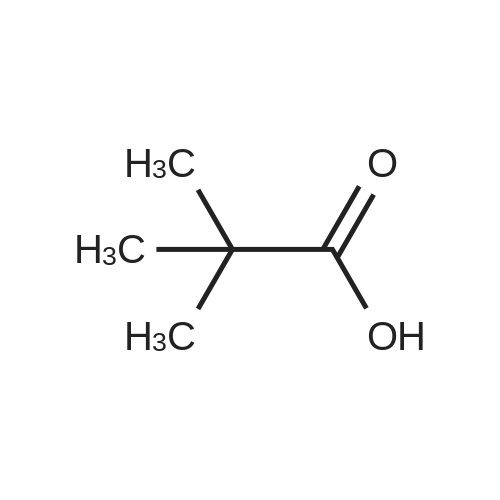
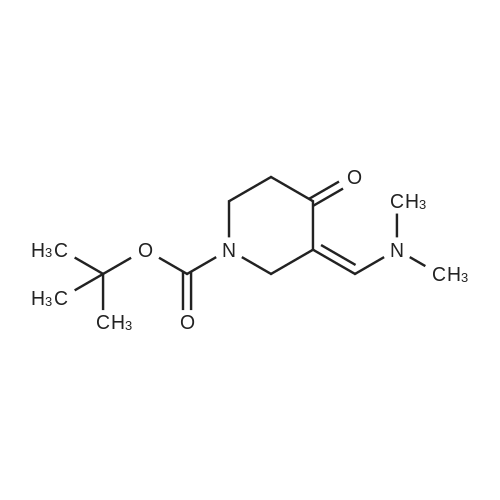
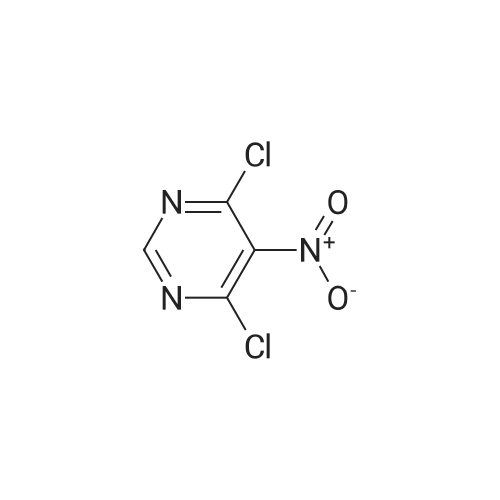
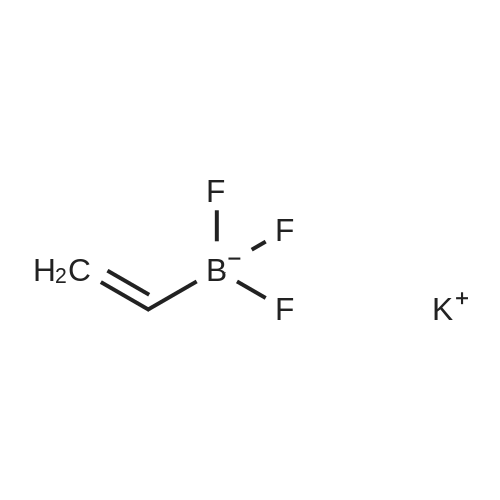
|
With 4-methyl-morpholine; sodium hydroxide; nitrogen; In water; N,N-dimethyl-formamide; |
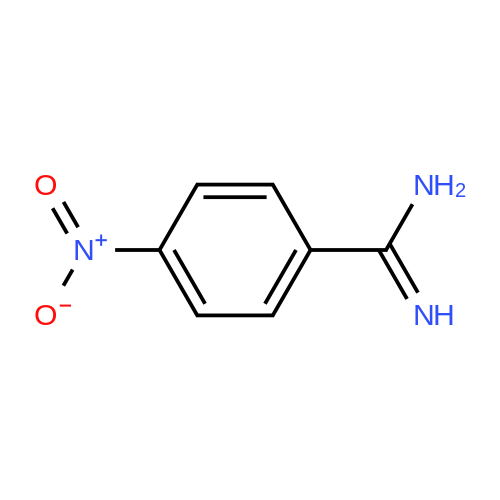
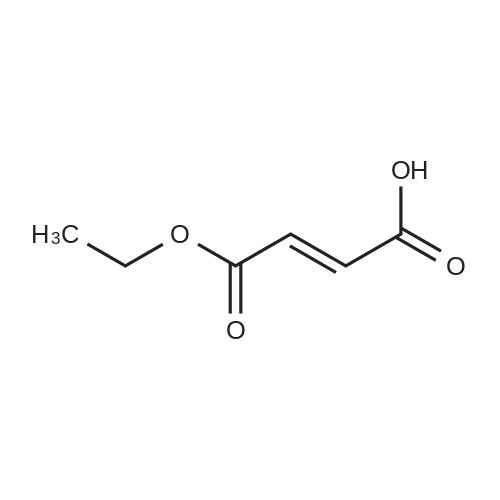
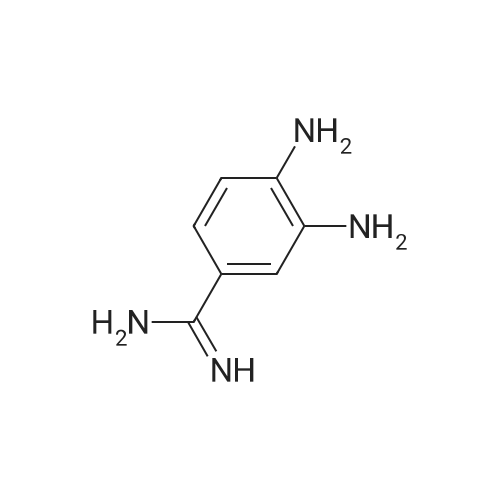
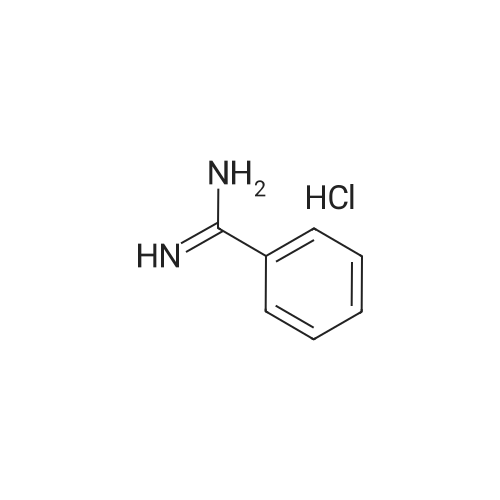
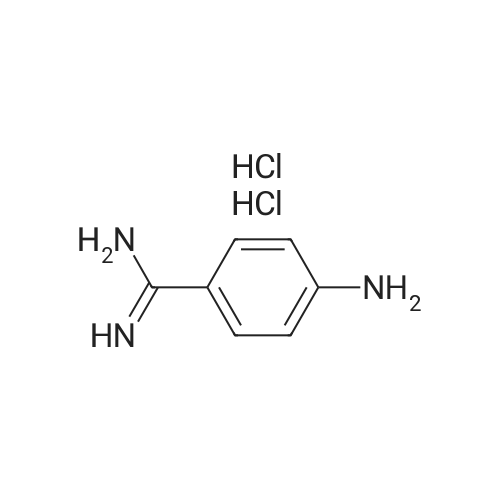
Step 1 Preparation of 4-[[4-(aminoiminomethyl)phenyl]amino]-4-oxo-buten-(E)-oic acid In a round bottomed flask under a static atmosphere of dry nitrogen were mixed 1.4 g of monoethyl fumarate, 1.36 g of isobutyl chloroformate and 1.01 g N-methylmorpholine in 100 mL DMF. <strong>[2498-50-2]4-aminobenzamidine dihydrochloride</strong> (2.06 g) and 2.02 g N-methylmorpholine were added at room temperature and the reaction mixture was stirred at 25 C. for 30 min. Water and sodium hydroxide were added to pH 10 and after one hour stirring neutralized to pH 7 to precipitate the zwitterion. Filtration provided 1 g of the desired compound as a white solid: 1 H NMR (d6 -DMSO) delta 1.1 (t, 3 H, J=7 Hz), 2.45 (m, 2H), 2.6 (m, 2H), 2.75 (d, 2H, J=7 Hz), 4.0 (q, 2H, J=7 Hz), 4.2 (dd, 1H, J=7 Hz and 8 Hz), 7.3 (m, 4H), 7. 8 (s, 4H), 8.45 (d, 1H, J=8 Hz), 9.05 (bs, 2H), 9.2 (bs, 2H), 10.4 (s, 1H). |
|
With 4-methyl-morpholine; sodium hydroxide; nitrogen; In water; N,N-dimethyl-formamide; |
Step 1 Preparation of 4-[[4-(aminoiminomethyl)phenyl]amino]-4-oxo-buten-(E)-oic acid In a round bottomed flask under a static atmosphere of dry nitrogen were mixed 1.4 g of monoethyl fumarate, 1.36 g of isobutyl chloroformate and 1.01 g N-methylmorpholine in 100 mL DMF. <strong>[2498-50-2]4-aminobenzamidine dihydrochloride</strong> (2.06 g) and 2.02 g N-methylmorpholine were added at room temperature and the reaction mixture was stirred at 25 C. for 30 min. Water and sodium hydroxide were added to pH 10 and after one hour stirring neutralized to pH 7 to precipitate the zwitterion. Filtration provided 1 g of the desired compound as a white solid: 1 H NMR (d6 -DMSO) delta 1.1 (t, 3 H, J=7 Hz), 2.45 (m, 2H), 2.6 (m, 2H), 2.75 (d, 2H, J =7 Hz), 4.0 (q, 2H, J=7 Hz), 4.2 (dd, 1H, J=7 Hz and 8 Hz), 7.3 (m, 4H), 7. 8 (s, 4H), 8.45 (d, 1H, J=8 Hz), 9.05 (bs, 2H), 9.2 (bs, 2H), 10.4 (s, 1H). |
|
With 4-methyl-morpholine; sodium hydroxide; nitrogen; In water; N,N-dimethyl-formamide; |
Step 1 Preparation of 4-[[4-(aminoiminomethyl)phenyl]amino]-4-oxobuten-(E)-oic acid In a round bottomed flask under a static atmosphere of dry nitrogen were mixed 1.4 g of monoethyl fumarate, 1.36 g of isobutyl chloroformate and 1.01 g N-methylmorpholine in 100 mL DMF. <strong>[2498-50-2]4-aminobenzamidine dihydrochloride</strong> (2.06 g) and 2.02 g N-methylmorpholine were added at room temperature and the reaction mixture was stirred at 25 C. for 30 min. Water and sodium hydroxide were added to pH 10 and after one hour of stirring the reaction was neutralized to pH 7 to precipitate the zwitterion. Filtration provided 1 g of the desired compound as a white solid: 1 H NMR (d6 -DMSO) delta 1.1 (t, 3H, J=7 Hz), 2.45 (m, 2H), 2.6 (m, 2H), 2.75 (d, 2H, J=7 Hz), 4.0 (q, 2H, J=7 Hz), 4.2 (dd, 1H, J=7 Hz and 8 Hz), 7.3 (m, 4H), 7.8 (s, 4H), 8.45 (d, 1H, J=8 Hz), 9.05 (bs, 2H), 9.2 (bs, 2H), 10.4 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping