Alternatived Products of [ 2271-72-9 ]
Product Details of [ 2271-72-9 ]
| CAS No. : | 2271-72-9 |
MDL No. : | MFCD09759165 |
| Formula : |
C9H9NO5
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
211.17
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 2271-72-9 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 2271-72-9 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 2271-72-9 ]
- 1
-
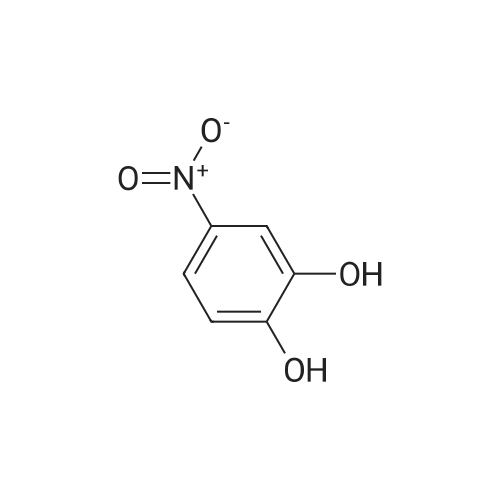 [ 3316-09-4 ]
[ 3316-09-4 ]

-
 [ 106-89-8 ]
[ 106-89-8 ]

-
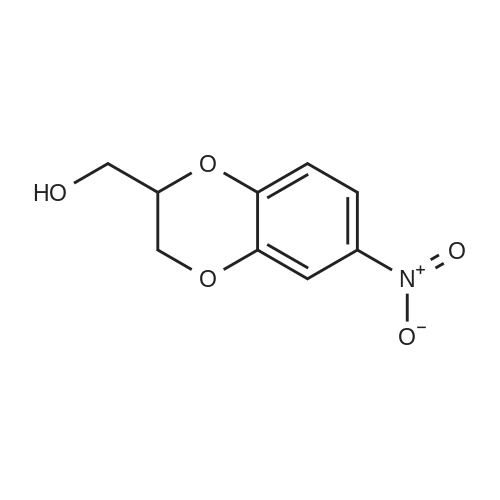 [ 2271-72-9 ]
[ 2271-72-9 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With sodium hydride; In N,N-dimethyl-formamide; at 0 - 80℃; |
Intermediate 59; Preparation of (6-Amino-2,3-dihydro-benzo[1,4]dioxin-2-yl)-methanol; (6-Nitro-2,3-dihydro-benzo[1,4]dioxin-2-yl)-methanol 1.93 g of 60% sodium hydride was suspended in 90 mL DMF. At 0 C. a solution of 5.15 g of <strong>[3316-09-4]4-nitrocatechol</strong> was added dropwise over 15 min. Subsequently, 3.9 g of epichlorohydrin in 10 mL DMF were added over 15 min. Stirring was continued at room temperature, then at 80 C. overnight. The mixture was diluted with water and extracted three times with ethyl acetate, dried (Na2SO4), filtered and concentrated under vacuum to give a yellow oil. The oil was purified by column chromatography on silica gel using a EtOAc-hexanes (0-100% gradient) to give the product (2.3 g) as a yellow solid. |
|
|
Intermediate 22 Preparation of (6-Amino-2,3-dihydro-benzo[1,4]dioxin-2-yl)-methanol; (6-Nitro-2,3-dihydro-benzo[1,4]dioxin-2-yl)-methanol 1.93g of 60% sodium hydride was suspended in 90 mL DMF. At 0 C. a solution of 5.15 g of <strong>[3316-09-4]4-nitrocatechol</strong> was added dropwise over 15 min. Subsequently, 3.9 g of epichlorohydrin in 10 mL DMF were added over 15 min. Stirring was continued at room temperature, then at 80 C. overnight. The mixture was diluted with water and extracted three times with ethyl acetate, dried (Na2SO4), filtered and concentrated under vacuum to give a yellow oil. The oil was purified by column chromatography on silica gel using a EtOAc-hexanes (0-100% gradient) to give the product (2.3 g) as a yellow solid. |
- 2
-
 [ 2271-72-9 ]
[ 2271-72-9 ]

-
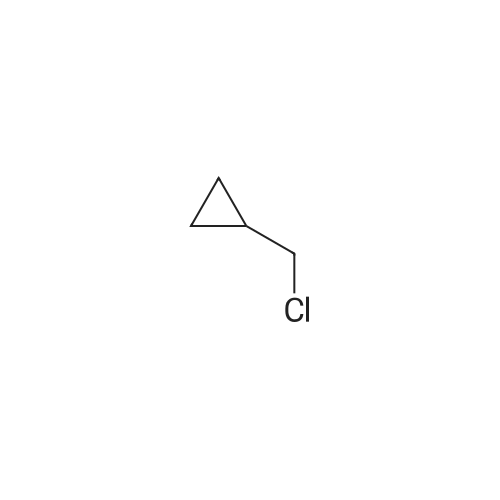 [ 5911-08-0 ]
[ 5911-08-0 ]

-
 [ 908248-15-7 ]
[ 908248-15-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 50% |
Stage #1: (6-nitro-2,3-dihydrobenzo[b][1,4]dioxin-2-yl)methanol With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.166667h;
Stage #2: chloro(cyclopropyl)methane In N,N-dimethyl-formamide at 0 - 20℃; for 0.333333h;
Stage #3: With tetrabutylammomium bromide In N,N-dimethyl-formamide at 20℃; |
4
Intermediate 4 Preparation of 2-((cyclopropylmethoxy)methyl)-2,3-dihydrobenzo[b][1,4]dioxin-6-amine 2-((cyclopropylmethoxy)methyl)-2,3-dihydro-6-nitrobenzo[b][1,4]dioxine; (2,3-dihydro-6-nitrobenzo[b][1,4]dioxin-2-yl)methanol (500 mg, 0.002 mol) and sodium hydride (0.28 g, 0.0070 mol) were placed in a flask under nitrogen. The flask was placed in an ice bath and 25 mL DMF was added. The reaction was stirred at 0° C. for 10 minutes and then (chloromethyl)cyclopropane (440 μL, 0.0048 mol) was added. The mixture was warmed to room temp over 20 min then tetra-N-butylammonium bromide (1.53 g, 0.00475 mol) was added to the mixture and the reaction was stirred at room temperature overnight. The reaction was partitioned between EtOAc and water. The organic layer was separated, washed with brine, dried (Na2SO4), filtered and the filtrate was concentrated under vacuum to an oil. The oil was purified by column chromatography on silica gel using EtOAc/hexanes (10%) as eluent to give a yellow solid (0.33 g, 50%) as a solid. m/z=266 (M+1). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







