|
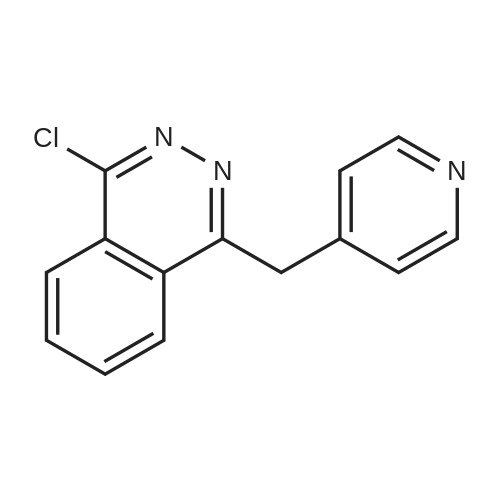
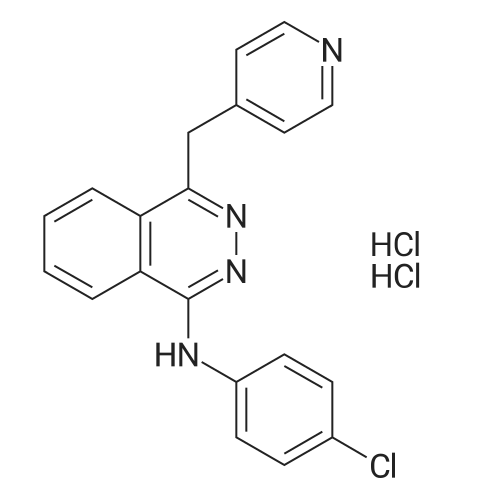
Stage #1: 4-chloro-aniline; 1-chloro-4-(pyridin-4-yl-methyl)-phthalazine In butan-1-ol for 2h; Heating / reflux;
Stage #2: With hydrogenchloride In methanol |
3a
A mixture of 15.22 g (59.52 mmol) l-chloro-4-(4- pyridylmethyl)phthalazine (for preparation see German Auslegeschriftno. 1 061 788 published JuI. 23, 1959]), 7.73 g (60.59 mmol) 4-chloroaniline and 200 ml 1-butanol is heated for 2 h under reflux. The crystallizate which is obtained when the mixture slowly cools to 5° C. is then filtered off and washed with 1-butanol and ether. The filter residue is dissolved in about 200 ml hot methanol, the solution is treated with 0.75 g activated carbon and filtered Via a Hyfio Super CeI, and the pH of the filtrate is adjusted to about 2.5 with 7 ml 3N methanolic HCl. The filtrate is evaporated to about half the original volume and ether added until slight turbidity occurs; cooling then leads to the precipitation of crystals. The crystallizate is filtered off, washed with a mixture of methanol/ether (1:2) as well as ether, dried for 8 h at 110° C. under HV, and equilibrated for 72 h at 20° C. and in room atmosphere. In this way, the title compound is obtained with a water content of 8.6%; m.p. >270° C. ; ' H NMR (DMSO-d 6 ) 11.05-12.20 (br), 9.18-9.23 (m, IH), 8. 88 (d, 2H), 8.35-8.40 (m, IH), 8.18-8.29 (m, 2H), 8.02 (d, 2H), 7.73 (d, 2H), 7.61 (d, 2H), 5.02 (s, 2H); ESI-MS: (M+H) + =347. |
|
In butan-1-ol for 2h; Heating / reflux; |
60
A mixture of 15.22 g (59.52 mmol) l-chloro-4-(4- pyridylmethyl)phthalazine (for preparation see German Auslegeschriftno. 1 061 788 published JuI. 23, 1959]), 7.73 g (60.59 mmol) 4-chloroaniline and 200 ml 1-butanol is heated for 2 h under reflux. The crystallizate which is obtained when the mixture slowly cools to 5° C. is then filtered off and washed with 1-butanol and ether. The filter residue is dissolved in about 200 ml hot methanol, the solution is treated with 0.75 g activated carbon and filtered Via a Hyflo Super CeI, and the pH of the filtrate is adjusted to about 2.5 with 7 ml 3N methanolic HCl. The filtrate is evaporated to about half the original volume and ether added until slight turbidity occurs; cooling then leads to the precipitation of crystals. The crystallizate is filtered off, washed with a mixture of methanol/ether (1 :2) as well as ether, dried for 8 h at 110° C. under HV, and equilibrated for 72 h at 20° C. and in room atmosphere. In this way, the title compound is obtained with a water content of 8.6%; m.p. >270° C j 1 H NMR (DMSO-d 6 ) 11.05-12.20 (br), 9.18-9.23 (m, IH), 8. 88 (d, 2H), 8.35-8.40 (m, IH), 8.18-8.29 (m, 2H), 8.02 (d, 2H), 7.73 (d, 2H), 7.61 (d, 2H), 5.02 (s, 2H); ESI-MS: (M+H) +=347. |
|
Stage #1: 4-chloro-aniline; 1-chloro-4-(pyridin-4-yl-methyl)-phthalazine In butan-1-ol for 2h; Heating / reflux;
Stage #2: With hydrogenchloride In methanol |
1
A mixture of 15.22 g (59.52 mmol) 1-chloro-4-(4-pyridylmethyl)phthalazine (for preparation see German Auslegeschrift no. 1 061 788 [published 23 July 1959]), 7.73 g (60.59 mmol) 4-chloroaniline and 200 ml 1-butanol is heated for 2 h under reflux. The crystallizate which is obtained when the mixture slowly cools to 5°C is then filtered off and washed with 1-butanol and ether. The filter residue is dissolved in about 200 ml hot methanol, the solution is treated with 0.75 g activated carbon and filtered via a Hyflo Super Cel, and the pH of the filtrate is adjusted to about 2.5 with 7 ml 3N methanolic HCl. The filtrate is evaporated to about half the original volume and ether added until slight turbidity occurs; cooling then leads to the precipitation of crystals. The crystallizate is filtered off, washed with a mixture of methanol / ether (1:2) as well as ether, dried for 8 h at 110°C under HV, and equilibrated for 72 h at 20°C and in room atmosphere. In this way, the title compound is obtained with a water content of 8.6%; m.p. >270 °C; 1 H NMR (DMSO-d6) 11.05-12.20 (br), 9.18-9.23 (m, 1 H), 8.88 (d, 2H), 8.35-8.40 (m, 1 H), 8.18-8.29 (m, 2H), 8.02 (d, 2H), 7.73 (d, 2H), 7.61 (d, 2H), 5.02 (s, 2H); ESI-MS: (M+H)+=347. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping