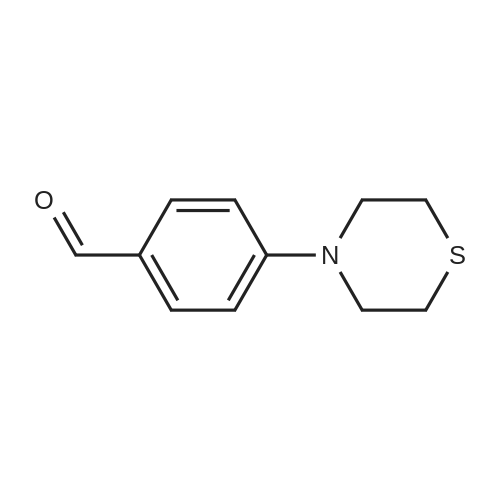
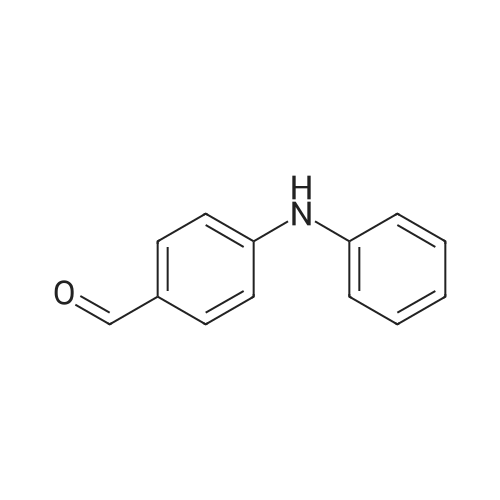
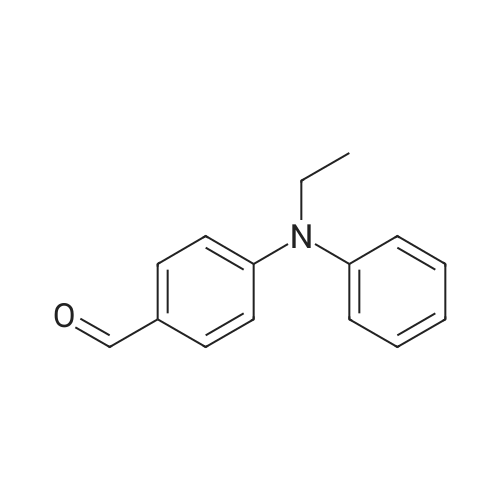
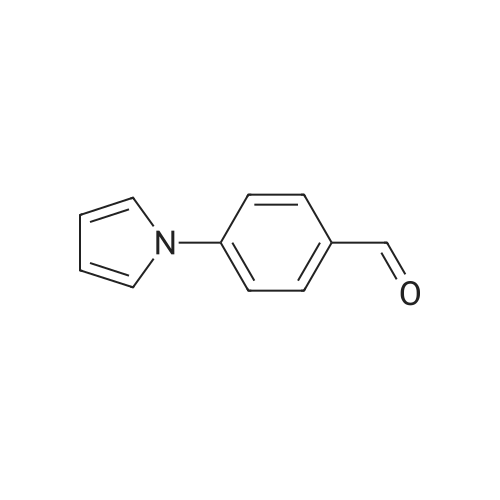
| 82% |
With phosphoric acid; In ethanol; at 0 - 50℃; |
20% H3PO4 in EtOH (0.02 mL) was added in the solution of oseltamivir free base (25 mg, 0.08 mmol) in EtOH (0.5 mL). The mixture was stirred and heated to 50 ?C for 30 min and then cooled to 0 ?C. The solvent was removed and the residue was purified by short column chromatography eluted with MeOH to gave oseltamivir phosphate as a white solid (27 mg, 82%). |
| 70% |
With phosphoric acid; In ethanol; at 60℃; for 3h; |
To a solution of 13 (10 mg) in ethanol (1 mL) and added a hot (60 C) solution of phosphoric acid (3 mg) in ethanol (1 mL). The reaction mixture was heated and stirred at 60 C for 3 h. After cooling to 0 C, the precipitates were collected by filtration and rinsed with cold acetone (2*1 mL) to afford oseltamivir phosphate (1) (9 mg, 70%) as a white crystal. |
| 7.6% |
With phosphoric acid; In ethanol; |
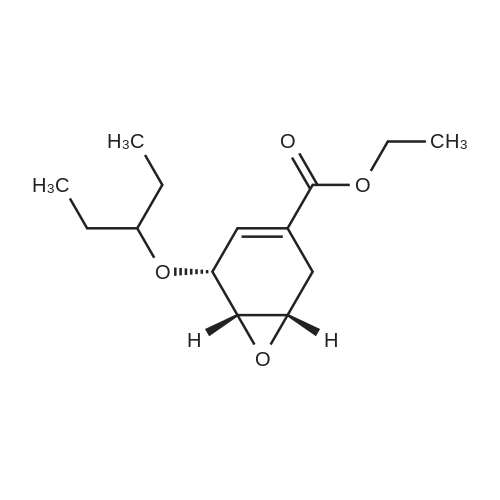
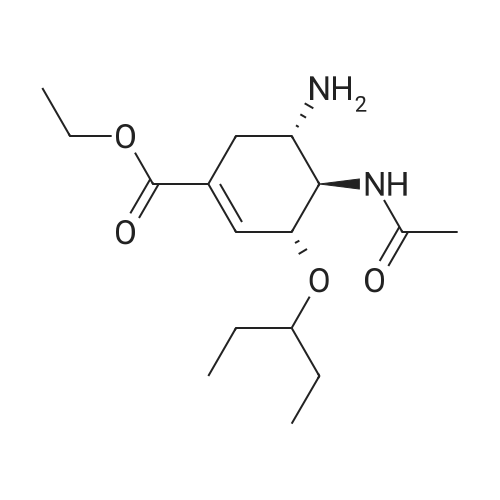
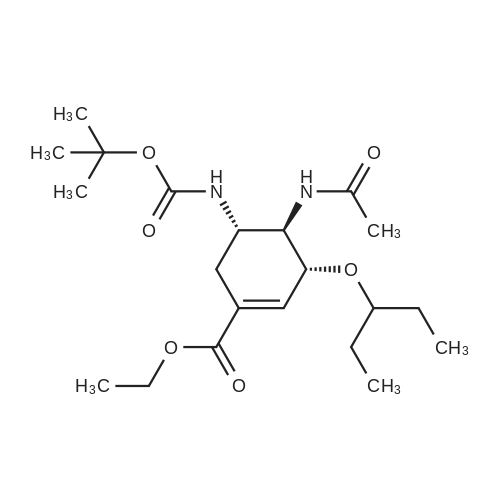
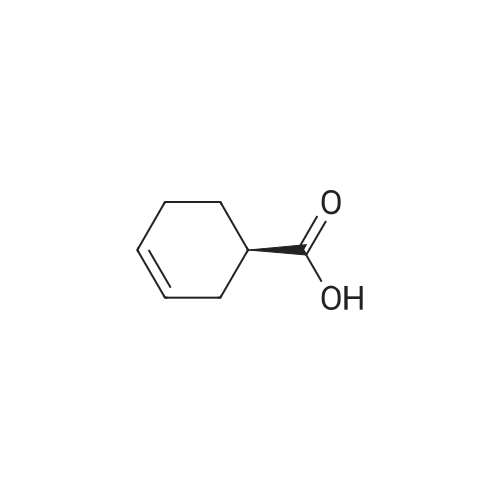
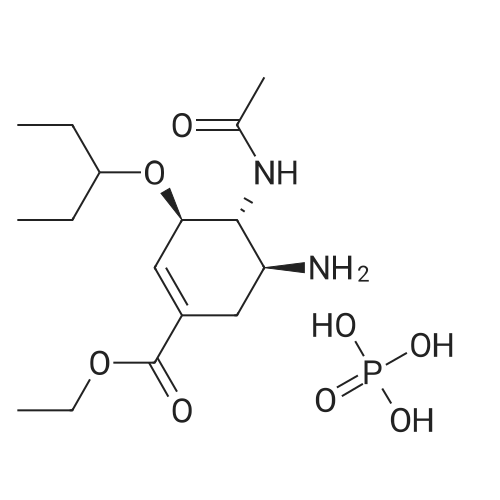
Example 13; [Show Image] As shown in the chemical equation 20, after the 1-cyclohexene-1-carboxylic acid derivative (26a) (18.9 mg, 60.5 mumol) was dissolved in ethanol (450 ml), phosphoric acid (ethanol 1 M solution, 60.5 ml) was added, and condensation was performed under reduced pressure, so that a crystal was obtained. This crystal was washed with ethyl acetate and was then recrystallized from ethanol. The obtained crystal was washed twice with acetone, so that a phosphate (27a) (3.3 mg, 8.0 mumol, Y. 7.6%) was obtained in the form of a white crystal. The spectrum data of this phosphate (27a) is as shown below and coincided with the spectrum data of an authentic sample of oseltamivir. In addition, the spectrum data of the compounds (25a) and (26a) coincided with that obtained from a compound derived from the authentic sample of oseltamivir. Furthermore, since the optical rotation of the compound (25a) coincided with that of a compound having an allyl carbamate group derived from the amino group at the 5-position of the authentic sample of oseltamivir, the absolute configuration represented by each chemical formula was confirmed. 1H NMR (D2O):delta(ppm) 6.91 (s, 1H), 4.38 (brd, J = 8.3 Hz, 1H), 4.31 (m, 2H), 4.11 (dd, J = 11.9, 9.5 Hz, 1H), 3.62 (m, 2H),3.02(dd, J = 17.2, 5.8 Hz, 1H), 2.58 (m, 1H), 2.14 (s, 3H), 1.70-1.45 (m, 4H), 1.34 (t, J = 7.0 Hz, 3H), 0.94 (t, J = 7.8 Hz, 3H), 0.90 (t, J = 7.8 Hz, 3H). |
|
With phosphoric acid; In ethanol; at 55℃;Product distribution / selectivity; |
A solution of azide 11a (170 mg, 0.5 mmol) in ethanol (20 niL) was treated with Lindlar's catalyst (100 mg) under an atmosphere of hydrogen for 16 h at room temperature. The reaction mixture was filtered through Celite, and rinsed with ethanol. The filtrate was evaporated under reduced pressure to give colorless foam (155 mg), which was dissolved in ethanol (3 mL) and added slowly in portions to a hot (550 C) solution of phosphoric acid (85%, 115 mg, 0.6 mmol) in ethanol (5 mL). Crystallization commenced within minutes. After cooling to o C, the precipitates were collected by filtration and rinsed with cold acetone (2 x) to afford 1 (187 mg, 91% yield). White crystal, mp 189-1910 C [lit. (Fukuta et al., J. Am. Chem. Soc. 2006,128, 6312-6313) mp 184-1860 C]; [alpha]D20 = -35.8 (c = 1, H2O) [lit.(Rohloff et al., . J. Org. Chem. 1998, 63, 4545-4550.) [alpha] = -39.9 (c = 1, H2O); or lit. (Fukuta et al., 2006) [alpha]D22 = -30.5 (c = 0.480, H2O)]; IR (neat) 3501, 1734, 1612, 1150 cm-i; 1H NMR (600 MHz, D2O) delta 6.91 (1 H, s), 4.39 (1 H, d, J =8.0 Hz), 4.32-4.30 (2 H, m), 4.11 (1 H, dd, J = 10.5, 5.7 Hz), 3-67"3-59 (2 H, m),3.01 (1 H, dd, J = 17.4, 5.4 Hz), 2.60-2.56 (1 H, m), 2.14 (3 H, s), 1.61-1.50 (4 H, m), 1.34 (3 H, t, J = 7.1 Hz), 0.94 (3 H, t, J = 7.3 Hz), 0.89 (3 H, t, J = 7.3 Hz); «C NMR (150 MHz, D2O) delta 178.1, 170.3, 140.7, 130.4, 87.2, 77.9, 65.2, 55.4, 52.0, 30.9, 28.3, 27.9, 25.2, 16.1, 11.36, 11.30; 3phi NMR (162 MHz, D2O) delta 0.43; HRMS calcd for CiOH29N2O4 (M+- H3PO4 + H): 313.2127, found: m/z 313.2123. Anal. Calcd for CiH3iN2theta8P: C, 46.83; H, 7.61; N, 6.83. Found: C, 46.70; H, 7.69; N, 6.74. |
|
With phosphoric acid; In ethyl acetate; acetone; at 20℃; for 4h;Heating / reflux;Product distribution / selectivity; |
Ethyl (3R,4R,5S)-4-(Acetylamino)-5-azido-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate (8.5 gm) was dissolved in tetrahydrofuran (130 ml) and then triphenyl phosphine (10.5 gm) and water (50 ml) are added. The contents were heated to reflux, refluxed for 5 hours and then distilled off the solvent under vacuum. To the reaction mass added ethyl acetate (80 ml), washed with 30% sodium chloride solution (50 ml) and distilled off the solvent completely under vacuum. Acetone (130 ml) was added to the residue, heated to reflux, under reflux the mixture of H3PO4 (3 gm) and ethyl acetate (50 ml) was slowly added during 1 hour and then refluxed for 1 hour. The reaction mass was cooled to 25 C. and stirred for 2 hours at 20-25 C. Filtered the solid, washed with acetone (10 ml) and dried at 60-65 C. for 4 hours to yield 6.5 gm of oseltamivir phosphate (HPLC Purity: 99.6%). |
|
With phosphoric acid; In ethanol; acetone; at 20℃; for 5.5h;Heating / reflux;Product distribution / selectivity; |
Ethyl (3R,4R,5S)-4-(Acetylamino)-5-azido-3-(1-ethylpropoxy)-1-cyclohex-ene-1-carboxylate (8.5 gm) was added to pyridine (200 ml) and then bubbled H2S gas for 3 hours at 25-35 C. Stopped the bubbling of H2S gas and then the reaction mixture was stirred for 5 hours at 25-35 C. The reaction mass was flushed with N2 gas for 20-30 minutes and distilled off the solvent completely under reduced pressure keeping the bath temperature below 50 C. To the residue added ethyl acetate (100 ml) and washed with 30% sodium chloride solution (50 ml). Distilled off the ethyl acetate completely under reduced pressure. Acetone (100 ml) was added to the residue, heated to reflux, under reflux the mixture of H3PO4 (3.2 gm) and ethanol (25 ml) was slowly added during 1 hour 30 minutes and then refluxed for 2 hours. The reaction mass was cooled to 25 C. and then stirred for 2 hours at 20-25 C. Filtered the solid, washed with acetone (10 ml) and dried at 60-65 C. for 4 hours to give 6.9 gm of oseltamivir phosphate (HPLC purity: 99.8%). |
| 997 mg |
With phosphoric acid; In ethanol; water; ethyl acetate; at 20 - 50℃; |
Compound 9 (0.90 g, 2.66 mmol) was dissolved in aqueous tetrahydrofuran (20 mL, THF/H2O = 10:1). Triphenylphosphine (0.77 g, 2.94 mmol) was added in portions at room temperature. After the mixture was heated at reflux and stirring was continued for 8 h. Solvents were removed by vacuum distillation, and the residue was cooled down to room temperature. The residue was then dissolved in a mixed solvent of ethyl acetate (3.5 mL) and ethanol (1.5 mL). After aqueous phosphoric acid (368 mg, 85% w/w, 3.19 mmol) was added, the mixture was warmed to 50 C, and stirring was continued for 2 h at 50 C. The reaction mixture was cooled down to room temperature and stirred for a further 8 h. White crystals were formed and precipitated. After suction and twice rinsing with ethyl acetate, the white crystals were dried under vacuum at 50 C overnight to furnish the title compound oseltamivir phosphate 1 (997 mg, 2.43 mmol) in 91% yield. The characterization data of compound 1 were identical with those of the sample obtained in our previous articles.31,32 |
| 0.34 g |
With phosphoric acid; In ethanol; ethyl acetate; at 50℃; for 2h; |
A solution of compound 8 (460.0mg, 1.005mmol) in DMSO (8mL) was heated to 80C. Fine powdered Cs2CO3 (815.0mg, 2.501mmol) was added quickly, and the mixture was stirred at 80C for 15min. An aqueous solution of acetic acid (50% w/w, 3mL) was added, and stirring was continued at 80C for 20min. After the mixture was cooled down to room temperature, ethyl acetate (30mL) and an aqueous solution of K2CO3 (20% w/w, 15mL) were added. After the mixture was vigorously stirred for 10min, the two phases were separated, and the aqueous phase was extracted twice with ethyl acetate (2×20mL). The organic extracts were combined and dried over anhydrous MgSO4. Evaporation of the solvent under vacuum gave a pale yellow oily residue, which was dissolved in a mixed solvent of ethyl acetate (4mL) and ethanol (4mL). Phosphoric acid (85%, 130.0mg, 1.128mmol) was then added, and the resulting suspension was heated and stirred at 50C for 2h. The suspension was cooled down to room temperature, and then allowed to stand overnight. White crystals were collected on a Buchner funnel by suction and washed twice with ethyl acetate (2×1mL). After being dried overnight in warm air, oseltamivir phosphate 1 (0.340g, 0.828mmol) was obtained in 82% yield. The characterization data of compound 1 were identical with those of the sample obtained in our previous report.(a), (b), (c)and(d) |
|
With phosphoric acid; In ethanol; acetone;Reflux; |
[0102] A phosphoric acid salt of the compound expressed by the Structural Formula (I) can be obtained in the following manner. Specifically, a solution of the compound expressed by the Structural Formula (I) in acetone is refluxed and treated with phosphoric acid in absolute ethanol. Crystallization commences immediately and after cooling to 0 C. for 12 hours the precipitate is collected by filtration to afford the phosphoric acid salt of the compound expressed by the Structural Formula (I) as colorless needles. |
|
With phosphoric acid; In ethanol; at 45 - 50℃; for 0.75h; |
The above formula (II) is dissolved in 240 ml of absolute ethanol at room temperature, and the temperature is raised to 45 to 50 C, and the addition is completed over 60 minutes.Dissolve a mixed solution of 13.4 g of 85% phosphoric acid in 150 ml of absolute ethanol, and stir at 45 to 50 C for 45 minutes; slowly cool down,4.0h was lowered to 0 C, stirred at -5 to 0 C for 60 minutes, suction filtered, and the filter cake was rinsed with 60 ml of absolute ethanol and drained. Vacuum drying Drying (45 ~ 50 C) for 6 h, obtained a white solid (I) crude. The crude product of formula (I) is dissolved in 180 ml of absolute ethanol + 30 ml of pure at room temperature. In a mixed solvent of water, the temperature is raised to 45 to 50 C, the temperature is maintained for 60 minutes, and the mixture is rapidly filtered while the mother liquor is at 45. Concentrated to a solid precipitate at C; add 180 ml of absolute ethanol, continue to concentrate at 45 C to a paste; add 180 ml of absolute ethanol, Warm up, maintain 45 ~ 50 C and continue to stir for 60 minutes; adjust the stirring speed to 75 rev / min, slowly cool down, 2.5 h will drop the temperature The mixture was stirred at -0 to 0 C for 120 minutes to 0 C; filtered, and the filter cake was rinsed with 60 ml of anhydrous ethanol and drained. Dry at 45~50C vacuum After drying for 6 hours, the white solid (I) oseltamivir phosphate was obtained.The total yield was 65.8% and the purity was 99.8%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping